Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Terephthalic acid,used in the production of polyester fibers and films,is composed of carbon,hydrogen,and oxygen.When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O.If its molar mass is between 158 and 167 g/mol,what is its molecular formula?
(Multiple Choice)
4.8/5  (27)
(27)
Sulfur dioxide reacts with chlorine to produce thionyl chloride (used as a drying agent for inorganic halides)and dichlorine oxide (used as a bleach for wood,pulp and textiles).
SO2(g)+ 2Cl2(g) SOCl2(g)+ Cl2O(g)
If 0.400 mol of Cl2 reacts with excess SO2,how many moles of Cl2O are formed?
(Multiple Choice)
4.9/5  (53)
(53)
In a correctly balanced equation,the number of reactant molecules must equal the number of product molecules.
(True/False)
4.9/5  (45)
(45)
Calculate the molar mass of tetraphosphorus decaoxide,P4O10,a corrosive substance which can be used as a drying agent.
(Multiple Choice)
4.8/5  (32)
(32)
Hydroxylamine nitrate contains 29.17 mass % N,4.20 mass % H,and 66.63 mass % O.Determine its empirical formula.
(Multiple Choice)
4.9/5  (33)
(33)
Aluminum will react with bromine to form aluminum bromide (used as an acid catalyst in organic synthesis).
Al(s)+ Br2(l) Al2Br6(s)[unbalanced]
How many moles of Al are needed to form 2.43 mol of Al2Br6?
(Multiple Choice)
4.9/5  (42)
(42)
Hydroxylamine nitrate contains 29.17 mass % N,4.20 mass % H,and 66.63 mass O.If its molar mass is between 94 and 98 g/mol,what is its molecular formula?
(Multiple Choice)
4.8/5  (36)
(36)
You are provided with a 250 mL volumetric flask,deionized water and solid NaOH.How much NaOH should be weighed out in order to make 250.mL of 0.100 M solution?
(Short Answer)
4.8/5  (37)
(37)
Terephthalic acid,used in the production of polyester fibers and films,is composed of carbon,hydrogen,and oxygen.When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O.What is its empirical formula?
(Multiple Choice)
4.7/5  (36)
(36)
Lead(II)nitrate is a poisonous substance which has been used in the manufacture of special explosives and as a sensitizer in photography.Calculate the mass of lead in 139 g of Pb(NO3)2.
(Multiple Choice)
4.8/5  (45)
(45)
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions)forms when aluminum reacts with oxygen.
4Al(s)+ 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 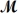 = 26.98 g/mol)and 117.65 g of oxygen (
= 26.98 g/mol)and 117.65 g of oxygen ( 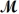 = 32.00 g/mol)is allowed to react.What mass of aluminum oxide (
= 32.00 g/mol)is allowed to react.What mass of aluminum oxide ( 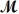 = 101.96 g/mol)can be formed?
= 101.96 g/mol)can be formed?
(Multiple Choice)
4.9/5  (33)
(33)
Magnesium (used in the manufacture of light alloys)reacts with iron(III)chloride to form magnesium chloride and iron.
3Mg(s)+ 2FeCl3(s) 3MgCl2(s)+ 2Fe(s)
A mixture of 41.0 g of magnesium ( 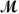 = 24.31 g/mol)and 175 g of iron(III)chloride (
= 24.31 g/mol)and 175 g of iron(III)chloride ( 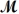 = 162.2 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 162.2 g/mol)is allowed to react.Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
(Multiple Choice)
4.9/5  (47)
(47)
Analysis of a white solid produced in a reaction between chlorine and phosphorus showed that it contained 77.44% chlorine and 22.56% phosphorus.What is its empirical formula?
(Short Answer)
4.7/5  (38)
(38)
For a sample consisting of 2.50 g of methane,CH4,calculate
a.the number of moles of methane present.
b.the total number of atoms present.
(Essay)
4.9/5  (40)
(40)
Balance the following equation:
C8H18O3(l)+ O2(g) H2O(g)+ CO2(g)
(Multiple Choice)
4.9/5  (43)
(43)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)