Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Gadolinium oxide,a colorless powder which absorbs carbon dioxide from the air,contains 86.76 mass % Gd.Determine its empirical formula.
(Multiple Choice)
4.9/5  (33)
(33)
In 0.20 mole of phosphoric acid,H3PO4,
a.how many H atoms are there?
b.what is the total number of atoms?
c.how many moles of O atoms are there?
(Essay)
4.8/5  (42)
(42)
Calcium fluoride,CaF2,is a source of fluorine and is used to fluoridate drinking water.Calculate its molar mass.
(Multiple Choice)
4.9/5  (37)
(37)
a.A solution of common salt,NaCl,in water has a concentration of 0.0921 M.Calculate the number of moles of HCl contained in 50.0 mL of this solution.
b.If,instead,an NaCl solution is prepared by dissolving 10.0 g of solid NaCl in enough water to make 250.mL of solution,what is the molarity?
(Essay)
4.8/5  (43)
(43)
Lead(II)sulfide was once used in glazing earthenware.It will also react with hydrogen peroxide to form lead(II)sulfate and water.How many grams of hydrogen peroxide are needed to react completely with 265 g of lead(II)sulfide?
(Multiple Choice)
4.8/5  (37)
(37)
Sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain.Calculate the mass in grams of 3.65 1020 molecules of SO3.
(Multiple Choice)
4.8/5  (43)
(43)
What volume,in L,of 10.0 M HCl is needed to make 2.00 L of 2.00 M HCl solution by dilution with water?
(Multiple Choice)
4.8/5  (28)
(28)
Alkanes are compounds of carbon and hydrogen with the general formula CnH2n+2.An alkane component of gasoline has a molar mass of between 125 and 130 g/mol.What is the value of n for this alkane?
(Multiple Choice)
4.8/5  (39)
(39)
When a solution is diluted with water,the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities.
(True/False)
4.9/5  (36)
(36)
Sodium hydroxide,also known as caustic soda,is used to neutralize acids and to treat cellulose in making of cellophane.Calculate the number of moles of solute in 1.875 L of 1.356 M NaOH solution.
(Multiple Choice)
4.7/5  (34)
(34)
Phosphine,an extremely poisonous and highly reactive gas,will react with oxygen to form tetraphosphorus decaoxide and water.
PH3(g)+ O2(g) P4O10(s)+ H2O(g)[unbalanced]
Calculate the mass of P4O10(s)formed when 225 g of PH3 reacts with excess oxygen.
(Multiple Choice)
4.9/5  (41)
(41)
Magnesium reacts with iron(III)chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants)and iron.
3Mg(s)+ 2FeCl3(s) 3MgCl2(s)+ 2Fe(s)
A mixture of 41.0 g of magnesium ( 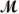 = 24.31 g/mol)and 175 g of iron(III)chloride (
= 24.31 g/mol)and 175 g of iron(III)chloride ( 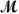 = 162.2 g/mol)is allowed to react.What mass of magnesium chloride = 95.21 g/mol)is formed?
= 162.2 g/mol)is allowed to react.What mass of magnesium chloride = 95.21 g/mol)is formed?
(Multiple Choice)
4.8/5  (43)
(43)
Balance the following equation:
B2O3(s)+ HF(l) BF3(g)+ H2O(l)
(Multiple Choice)
4.8/5  (39)
(39)
Gaseous methanol (CH4O)reacts with oxygen gas to produce carbon dioxide gas and liquid water.Write a balanced equation for this process.
(Essay)
4.8/5  (44)
(44)
When 2.61 g of solid Na2CO3 is dissolved in sufficient water to make 250 mL of solution,the concentration of Na2CO3 is:
(Multiple Choice)
4.8/5  (36)
(36)
What will be the final volume of a solution prepared by diluting 25 mL of 8.25 M sodium hydroxide to a concentration of 2.40 M?
(Multiple Choice)
4.8/5  (38)
(38)
Magnesium fluoride is used in the ceramics and glass industry.What is the mass of 1.72 mol of magnesium fluoride?
(Multiple Choice)
4.9/5  (37)
(37)
How many mL of concentrated nitric acid (HNO3,16.0 M)should be diluted with water in order to make 2.00 L of 2.00 M solution?
(Multiple Choice)
4.8/5  (39)
(39)
Aluminum oxide,Al2O3,is used as a filler for paints and varnishes as well as in the manufacture of electrical insulators.Calculate the number of moles in 47.51 g of Al2O3.
(Multiple Choice)
4.8/5  (33)
(33)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)