Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Which of the following samples has the most moles of the compound?
(Multiple Choice)
4.8/5  (41)
(41)
Gadolinium oxide, a colorless powder which absorbs carbon dioxide from the air, contains 86.76 mass % Gd. Determine its empirical formula.
(Multiple Choice)
4.7/5  (31)
(31)
Consider the balanced equation:
Al2S3(s) + 6H2O(l) 2Al(OH)3(s) + 3H2S(g)
If 15.0g of aluminum sulfide and 10.0g of water are allowed to react as above, and assuming a complete reaction
a. by calculation, find out which is the limiting reagent.
b. calculate the maximum mass of H2S which can be formed from these reagents.
c. calculate the mass of excess reagent remaining after the reaction is complete.
(Essay)
4.8/5  (35)
(35)
Constitutional (structural) isomers have the same empirical formula but different molecular formulas.
(True/False)
4.9/5  (35)
(35)
Aluminum will react with bromine to form aluminum bromide (used as an acid catalyst in organic synthesis). Al(s) + Br2(l) Al2Br6(s) [unbalanced]
How many moles of Al are needed to form 2.43 mol of Al2Br6?
(Multiple Choice)
4.8/5  (38)
(38)
Balance the following equation:
B2O3(s) + HF(l) BF3(g) + H2O(l)
(Multiple Choice)
4.8/5  (41)
(41)
Calcium fluoride, CaF2, is a source of fluorine and is used to fluoridate drinking water. Calculate its molar mass.
(Multiple Choice)
4.8/5  (36)
(36)
A compound containing chromium and silicon contains 73.52 mass percent chromium. Determine its empirical formula.
(Multiple Choice)
4.9/5  (39)
(39)
Determine the percent composition of potassium dichromate, K2Cr2O7.
(Multiple Choice)
4.8/5  (32)
(32)
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 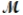 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 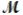 = 32.00 g/mol) is allowed to react. What mass of aluminum oxide (
= 32.00 g/mol) is allowed to react. What mass of aluminum oxide ( 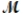 = 101.96 g/mol) can be formed?
= 101.96 g/mol) can be formed?
(Multiple Choice)
4.9/5  (34)
(34)
A compound of bromine and fluorine is used to make UF6, which is an important chemical in processing and reprocessing of nuclear fuel. The compound contains 58.37 mass percent bromine. Determine its empirical formula.
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the molar mass of tetraphosphorus decaoxide, P4O10, a corrosive substance which can be used as a drying agent.
(Multiple Choice)
4.7/5  (44)
(44)
Magnesium fluoride is used in the ceramics and glass industry. What is the mass of 1.72 mol of magnesium fluoride?
(Multiple Choice)
4.9/5  (44)
(44)
How many grams of sodium fluoride (used in water fluoridation and manufacture of insecticides) are needed to form 485 g of sulfur tetrafluoride?
3SCl2(l) + 4NaF(s) SF4(g) + S2Cl2(l) + 4NaCl(s)
(Multiple Choice)
4.9/5  (44)
(44)
Alkanes are compounds of carbon and hydrogen with the general formula CnH2n+2. An alkane component of gasoline has a molar mass of between 125 and 130 g/mol. What is the value of n for this alkane?
(Multiple Choice)
4.8/5  (38)
(38)
Hydroxylamine nitrate contains 29.17 mass % N, 4.20 mass % H, and 66.63 mass % O. Determine its empirical formula.
(Multiple Choice)
4.8/5  (37)
(37)
Balance the following equation:
UO2(s) + HF(l) UF4(s) + H2O(l)
(Multiple Choice)
4.8/5  (40)
(40)
Aluminum metal reacts with chlorine gas to form solid aluminum trichloride, AlCl3. What mass of chlorine gas is needed to react completely with 163 g of aluminum?
(Multiple Choice)
4.8/5  (41)
(41)
Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent, desiccant, or catalyst for organic reactions. 4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 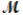 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 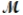 = 32.00 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 32.00 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)