Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
How many grams of oxygen are needed to react completely with 200.0 g of ammonia, NH3? 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide, Mg(OH)2.
(Multiple Choice)
4.8/5  (31)
(31)
Hydroxylamine hydrochloride is a powerful reducing agent which is used as a polymerization catalyst. It contains 5.80 mass % H, 20.16 mass % N, 23.02 mass % O, and 51.02 mass % Cl. What is its empirical formula?
(Multiple Choice)
4.8/5  (31)
(31)
Potassium dichromate, K2Cr2O7, is used in tanning leather, decorating porcelain, and water proofing fabrics. Calculate the number of chromium atoms in 78.82 g of K2Cr2O7.
(Multiple Choice)
4.9/5  (37)
(37)
Balance the following equation for the combustion of butane, a hydrocarbon used in gas lighters:
C4H10(g) + O2(g) CO2(g) + H2O(l)
(Essay)
4.9/5  (34)
(34)
A normal breath takes in about 1.0 L of air. Assuming that air has an average molar mass of 28.8 g, and that its density is 0.97 g/L, how many molecules of air do you take in with each breath?
(Multiple Choice)
4.7/5  (29)
(29)
The insecticide DDT was formerly in widespread use, but now it is severely restricted owing to its adverse environmental effects. It is prepared as follows:
C2HCl3O + 2C6H5Cl C14H9Cl5 + H2O
chloral chlorobenzene DDT
If 10.00 g of chloral were reacted with 10.00 g of chlorobenzene
a. what is the maximum amount (mol) of DDT which could be formed?
b. what is the limiting reagent?
c. what is the % yield, if 12.15 g of DDT is produced?
(Essay)
4.8/5  (32)
(32)
In 0.20 mole of phosphoric acid, H3PO4
a. how many H atoms are there?
b. what is the total number of atoms?
c. how many moles of O atoms are there?
(Essay)
4.7/5  (41)
(41)
Ammonia, NH3, is produced industrially from nitrogen and hydrogen as follows:
N2(g) + 3H2(g) 2NH3(g)
What mass, of which starting material, will remain when 30.0 g of N2 and 10.0 g of H2 react until the limiting reagent is completely consumed?
(Short Answer)
4.8/5  (42)
(42)
Ammonia, an important source of fixed nitrogen that can be metabolized by plants, is produced using the Haber process in which nitrogen and hydrogen combine.
N2(g) + 3H2(g) 2NH3(g)
How many grams of nitrogen are needed to produce 325 grams of ammonia?
(Multiple Choice)
4.8/5  (34)
(34)
In combustion analysis, the carbon and hydrogen contents of a substance are determined from the CO2 and H2O, respectively, which are collected in the absorbers.
(True/False)
4.9/5  (43)
(43)
Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. 3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 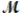 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 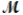 = 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
(Multiple Choice)
4.9/5  (38)
(38)
What is the mass in grams of 0.250 mol of the common antacid calcium carbonate?
(Multiple Choice)
4.8/5  (28)
(28)
For a sample consisting of 2.50 g of methane, CH4, calculate
a. the number of moles of methane present.
b. the total number of atoms present.
(Essay)
4.8/5  (33)
(33)
In combustion analysis, the oxygen content of a substance is equal to the total oxygen in the CO2 and H2O collected in the absorbers.
(True/False)
4.8/5  (37)
(37)
Gaseous methanol (CH4O) reacts with oxygen gas to produce carbon dioxide gas and liquid water. Write a balanced equation for this process.
(Essay)
4.8/5  (43)
(43)
Lead(II) sulfide was once used in glazing earthenware. It will also react with hydrogen peroxide to form lead(II) sulfate and water. How many grams of hydrogen peroxide are needed to react completely with 265 g of lead(II) sulfide?
(Multiple Choice)
4.8/5  (40)
(40)
Terephthalic acid, used in the production of polyester fibers and films, is composed of carbon, hydrogen, and oxygen. When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O. What is its empirical formula?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 61 - 80 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)