Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Balance the following equation for the combustion of benzene:
C6H6(l) + O2(g) H2O(g) + CO2(g)
(Multiple Choice)
4.8/5  (37)
(37)
Consider the balanced equation for the combustion of propane, C3H8
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(l)
If propane reacts with oxygen as above
a. what is the limiting reagent in a mixture containing 5.00 g of C3H8 and 10.0 g of O2?
b. what mass of CO2 is formed when 1.00 g of C3H8 reacts completely?
(Essay)
4.9/5  (44)
(44)
Phosphorus pentachloride, PCl5, a white solid that has a pungent, unpleasant odor, is used as a catalyst for certain organic reactions. Calculate the number of moles in 38.7 g of PCl5.
(Multiple Choice)
4.9/5  (24)
(24)
Potassium chlorate (used in fireworks, flares, and safety matches) forms oxygen and potassium chloride when heated. KClO3(s) KCl(s) + O2(g) [unbalanced]
How many grams of oxygen are formed when 26.4 g of potassium chlorate is heated?
(Multiple Choice)
4.9/5  (36)
(36)
What is the percent yield for the reaction PCl3(g) + Cl2(g) PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 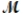 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
(Multiple Choice)
4.8/5  (38)
(38)
The iodine "clock reaction" involves the following sequence of reactions occurring in a reaction mixture in a single beaker.
1) IO3-(aq) + 5I-(aq) + 6H+(aq) 3I2(aq) + 3H2O(l)
2) I2(aq) + 2S2O32-(aq) 2I-(aq) + S4O62-(aq)
The molecular iodine (I2) formed in reaction 1 is immediately used up in reaction 2, so that no iodine accumulates. In one experiment, a student made up a reaction mixture which initially contained 0.0020 mol of iodate ions (IO3-). If the iodate ions reacted completely, how many moles of thiosulfate ions (S2O32-) were needed in reaction 2, in order to react completely with the iodine (I2) produced in reaction 1?
(Multiple Choice)
4.9/5  (46)
(46)
Showing 81 - 87 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)