Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Hydroxylamine nitrate contains 29.17 mass % N, 4.20 mass % H, and 66.63 mass O. If its molar mass is between 94 and 98 g/mol, what is its molecular formula?
(Multiple Choice)
4.8/5  (34)
(34)
Aluminum sulfate, Al2(SO4)3, is used in tanning leather, purifying water, and manufacture of antiperspirants. Calculate its molar mass.
(Multiple Choice)
4.7/5  (42)
(42)
An important reaction sequence in the industrial production of nitric acid is the following: N2(g) + 3H2(g) 2NH3(g)
4NH3(g) + 5O2(g) 4NO(g) + 6H2O(l)
Starting from 20.0 mol of nitrogen gas in the first reaction, how many moles of oxygen gas are required in the second one?
(Multiple Choice)
4.8/5  (36)
(36)
Ammonia will react with fluorine to produce dinitrogen tetrafluoride and hydrogen fluoride (used in production of aluminum, in uranium processing, and in frosting of light bulbs). 2NH3(g) + 5F2(g) N2F4(g) + 6HF(g)
How many moles of NH3 are needed to react completely with 13.6 mol of F2?
(Multiple Choice)
4.9/5  (36)
(36)
Propane, C3H8, is commonly provided as a bottled gas for use as a fuel. In 0.200 mol of propane
a. what is the mass of propane?
b. what mass of carbon is present?
c. how many molecules of C3H8 are present?
d. how many hydrogen atoms are present?
(Essay)
4.8/5  (40)
(40)
Tetraphosphorus hexaoxide ( 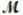 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
(Multiple Choice)
4.8/5  (51)
(51)
Balance the following equation:
Ca3(PO4)2(s) + SiO2(s) + C(s) CaSiO3(s) + CO(g) + P4(s)
(Multiple Choice)
4.8/5  (37)
(37)
Sulfur trioxide can react with atmospheric water vapor to form sulfuric acid that falls as acid rain. Calculate the mass in grams of 3.65 × 1020 molecules of SO3.
(Multiple Choice)
4.8/5  (33)
(33)
In the combustion analysis of 0.1127 g of glucose (C6H12O6), what mass, in grams, of CO2 would be produced?
(Multiple Choice)
4.8/5  (47)
(47)
How many atoms are in a drop of mercury that has a diameter of 1.0 mm? (Volume of a sphere is 4r3/3; density of mercury = 13.6 g/cm3)
(Multiple Choice)
4.9/5  (39)
(39)
Terephthalic acid, used in the production of polyester fibers and films, is composed of carbon, hydrogen, and oxygen. When 0.6943 g of terephthalic acid was subjected to combustion analysis it produced 1.471 g CO2 and 0.226 g H2O. If its molar mass is between 158 and 167 g/mol, what is its molecular formula?
(Multiple Choice)
5.0/5  (43)
(43)
Aluminum oxide, Al2O3, is used as a filler for paints and varnishes as well as in the manufacture of electrical insulators. Calculate the number of moles in 47.51 g of Al2O3.
(Multiple Choice)
4.8/5  (39)
(39)
Analysis of a white solid produced in a reaction between chlorine and phosphorus showed that it contained 77.44% chlorine and 22.56% phosphorus. What is its empirical formula?
(Short Answer)
5.0/5  (37)
(37)
Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 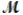 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 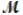 = 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
= 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
(Multiple Choice)
4.9/5  (41)
(41)
Balance the following equation for partial oxidation of ammonia, an important reaction in the production of nitric acid:
NH3(g) + O2(g) NO(g) + H2O(l)
(Essay)
4.9/5  (36)
(36)
Lead (II) nitrate is a poisonous substance which has been used in the manufacture of special explosives and as a sensitizer in photography. Calculate the mass of lead in 139 g of Pb(NO3)2.
(Multiple Choice)
4.8/5  (39)
(39)
Constitutional (structural) isomers have the same molecular formula but different structural formulas.
(True/False)
4.7/5  (39)
(39)
Showing 21 - 40 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)