Exam 17: Equilibrium: the Extent of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Ammonia is synthesized in the Haber process:
N2(g) + 3H2(g)  2NH3(g)
Kp for this reaction is 1.49 × 10-5 at 500.°C. Calculate Kc at this temperature.
2NH3(g)
Kp for this reaction is 1.49 × 10-5 at 500.°C. Calculate Kc at this temperature.
(Short Answer)
4.7/5  (30)
(30)
If Q > K, more products need to be formed as the reaction proceeds to equilibrium.
(True/False)
4.9/5  (39)
(39)
What is the mass-action expression, Qp, for the following reaction? SbF5(g) + 4Cl2(g)  SbCl3(g) + 5ClF(g)
SbCl3(g) + 5ClF(g)
(Multiple Choice)
4.9/5  (37)
(37)
The equilibrium constant Kc for the reaction PCl3(g) + Cl2(g)  PCl5(g)
Is 49 at 230°C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230°C, what is the concentration of PCl3 when equilibrium has been established?
PCl5(g)
Is 49 at 230°C. If 0.70 mol of PCl3 is added to 0.70 mol of Cl2 in a 1.00-L reaction vessel at 230°C, what is the concentration of PCl3 when equilibrium has been established?
(Multiple Choice)
4.8/5  (43)
(43)
Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 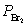 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
(Multiple Choice)
4.9/5  (34)
(34)
In a chemical reaction, if the starting concentrations of reactants are increased, then the equilibrium constant Kc will also increase.
(True/False)
4.7/5  (35)
(35)
Sodium hydrogen carbonate decomposes above 110°C to form sodium carbonate, water, and carbon dioxide. 2NaHCO3(s)  Na2CO3(s) + H2O(g) + CO2(g)
One thousand grams of sodium hydrogen carbonate are added to a reaction vessel, the temperature is increased to 200°C, and the system comes to equilibrium. What happens in this system if another 50 g of sodium carbonate are now added?
Na2CO3(s) + H2O(g) + CO2(g)
One thousand grams of sodium hydrogen carbonate are added to a reaction vessel, the temperature is increased to 200°C, and the system comes to equilibrium. What happens in this system if another 50 g of sodium carbonate are now added?
(Multiple Choice)
4.8/5  (41)
(41)
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product, phosgene (COCl2) CO(g) + Cl2(g) ![Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product, phosgene (COCl<sub>2</sub>) CO(g) + Cl<sub>2</sub>(g) <sub> </sub> COCl<sub>2</sub>(g) If the equilibrium constant for this reaction is K<sub>c</sub> = 248, predict, if possible, what will happen when the reactants and product are combined with the concentrations shown. [CO] = [Cl<sub>2</sub>] = 0.010 M; [COCl<sub>2</sub>] = 0.070 M](https://storage.examlex.com/TB5833/11ea8ef8_2b81_29d6_ab0a_897fdd8513da_TB5833_11.jpg) COCl2(g)
If the equilibrium constant for this reaction is Kc = 248, predict, if possible, what will happen when the reactants and product are combined with the concentrations shown.
[CO] = [Cl2] = 0.010 M; [COCl2] = 0.070 M
COCl2(g)
If the equilibrium constant for this reaction is Kc = 248, predict, if possible, what will happen when the reactants and product are combined with the concentrations shown.
[CO] = [Cl2] = 0.010 M; [COCl2] = 0.070 M
(Multiple Choice)
4.7/5  (38)
(38)
Write the mass-action expression, Qc, for the following chemical reaction equation. 2C6H6(g) + 15O2(g)  12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)
(Multiple Choice)
4.7/5  (44)
(44)
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) + O2(g)  2CO(g).
When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction?
2CO(g).
When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction?
(Multiple Choice)
5.0/5  (40)
(40)
The reaction system CS2(g) + 4H2(g)  CH4(g) + 2H2S(g)
Is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
CH4(g) + 2H2S(g)
Is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
(Multiple Choice)
4.9/5  (33)
(33)
The following reaction is at equilibrium at a pressure of 1 atm, in a closed container. NaOH(s) + CO2(g)  NaHCO3(s) H°rxn < 0
Which, if any, of the following actions will decrease the concentration of CO2 gas present at equilibrium?
NaHCO3(s) H°rxn < 0
Which, if any, of the following actions will decrease the concentration of CO2 gas present at equilibrium?
(Multiple Choice)
4.9/5  (34)
(34)
Stearic acid, nature's most common fatty acid, dimerizes when dissolved in hexane: 2C17H35COOH  (C17H35COOH)2 H°rxn = -172 kJ
The equilibrium constant for this reaction at 28°C is 2900. Estimate the equilibrium constant at 38°C.
(C17H35COOH)2 H°rxn = -172 kJ
The equilibrium constant for this reaction at 28°C is 2900. Estimate the equilibrium constant at 38°C.
(Multiple Choice)
4.8/5  (44)
(44)
Write the mass-action expression, Qc , for the following chemical reaction. Zn(s) + 2Ag+(aq)  Zn2+(aq) + 2Ag(s)
Zn2+(aq) + 2Ag(s)
(Multiple Choice)
4.8/5  (25)
(25)
A good catalyst for a reaction will speed up the forward reaction and slow down the reverse reaction.
(True/False)
4.8/5  (43)
(43)
The following reaction, in CCl4 solvent, has been studied at 25°C. 2BrCl  Br2 + Cl2
The equilibrium constant Kc is known to be 0.141. If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M, what is the equilibrium concentration of bromine?
Br2 + Cl2
The equilibrium constant Kc is known to be 0.141. If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M, what is the equilibrium concentration of bromine?
(Multiple Choice)
4.8/5  (40)
(40)
Hydrogen iodide, HI, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are heated together. If 20.0 g of hydrogen and 20.0 g of iodine are heated, forming 10.0 g of hydrogen iodide, what mass of hydrogen remains unreacted?
(Multiple Choice)
4.8/5  (43)
(43)
The equilibrium constant for reaction (1) below is 276. Under the same conditions, what is the equilibrium constant of reaction (2)? (1) 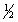 X2(g) +
X2(g) + 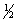 Y2(g)
Y2(g)  XY(g)
(2) 2XY(g)
XY(g)
(2) 2XY(g)  X2(g) + Y2(g)
X2(g) + Y2(g)
(Multiple Choice)
4.9/5  (37)
(37)
SO2 reacts with O2 to produce SO3. If 86.0 g of SO2 is placed in a reaction vessel along with excess oxygen gas, how many moles of SO2 remain when 50.0 g of SO3 have been formed?
(Multiple Choice)
5.0/5  (42)
(42)
At 450°C, tert-butyl alcohol decomposes into water and isobutene. (CH3)3COH(g)  (CH3)2CCH2(g) + H2O(g)
A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
(CH3)2CCH2(g) + H2O(g)
A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 41 - 60 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)