Exam 17: Equilibrium: the Extent of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Magnesium hydroxide is used in several antacid formulations. When it is added to water it dissociates into magnesium and hydroxide ions. Mg(OH)2(s)  Mg2+(aq) + 2OH-(aq)
The equilibrium constant at 25°C is 8.9 × 10-12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH)2 are now added to the mixture?
Mg2+(aq) + 2OH-(aq)
The equilibrium constant at 25°C is 8.9 × 10-12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH)2 are now added to the mixture?
(Multiple Choice)
4.8/5  (30)
(30)
The equilibrium constant, Kp , for the reaction CO(g) + H2O(g)  CO2(g) + H2(g)
At 986°C is 0.63. A rigid cylinder at that temperature contains 1.2 atm of carbon monoxide, 0.20 atm of water vapor, 0.30 atm of carbon dioxide, and 0.27 atm of hydrogen. Is the system at equilibrium?
CO2(g) + H2(g)
At 986°C is 0.63. A rigid cylinder at that temperature contains 1.2 atm of carbon monoxide, 0.20 atm of water vapor, 0.30 atm of carbon dioxide, and 0.27 atm of hydrogen. Is the system at equilibrium?
(Multiple Choice)
5.0/5  (30)
(30)
Compounds A, B, and C react according to the following equation. 3A(g) + 2B(g) ![Compounds A, B, and C react according to the following equation. 3A(g) + 2B(g) 2C(g) At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of K<sub>c</sub> for this reaction?](https://storage.examlex.com/TB5833/11ea8ef8_2b89_1885_ab0a_9369eccf12a7_TB5833_11.jpg) 2C(g)
At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of Kc for this reaction?
2C(g)
At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M, [B] = 1.23 M, and [C] = 1.75 M. What is the value of Kc for this reaction?
(Multiple Choice)
4.7/5  (34)
(34)
Hydrogen sulfide will react with water as shown in the following reactions. H2S(g) + H2O(l)  H3O+(aq) + HS-(aq) K1 = 1.0 × 10-7
HS-(aq) + H2O(l)
H3O+(aq) + HS-(aq) K1 = 1.0 × 10-7
HS-(aq) + H2O(l)  H3O+(aq) + S2-(aq) K2 = ?
H2S(g) + 2H2O(l)
H3O+(aq) + S2-(aq) K2 = ?
H2S(g) + 2H2O(l)  2H3O+(aq) + S2-(aq) K3 = 1.3 × 10-20
What is the value of K2?
2H3O+(aq) + S2-(aq) K3 = 1.3 × 10-20
What is the value of K2?
(Multiple Choice)
4.7/5  (37)
(37)
The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature. Br2(g) + Cl2(g)  2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g)
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g) 
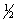 Br2(g) +
Br2(g) + 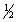 Cl2(g)
Cl2(g)
(Multiple Choice)
4.8/5  (39)
(39)
For a gas-phase equilibrium, a change in the pressure of any single reactant or product will change Kp.
(True/False)
4.8/5  (39)
(39)
Changing the amount of a solid reactant or product in an equilibrium reaction will not affect the amounts of the other reactants and products present at equilibrium.
(True/False)
4.8/5  (34)
(34)
The reaction quotient, Qc, for a reaction has a value of 75 while the equilibrium constant, Kc, has a value of 195. Which of the following statements is accurate?
(Multiple Choice)
4.7/5  (30)
(30)
Nitrogen dioxide decomposes according to the reaction 2NO2(g)  2NO(g) + O2(g)
Where Kp = 4.48 × 10-13 at 25°C. What is the value for Kc?
2NO(g) + O2(g)
Where Kp = 4.48 × 10-13 at 25°C. What is the value for Kc?
(Multiple Choice)
4.8/5  (36)
(36)
Consider the equilibrium reaction shown below. B2(g)  2B(g)
If the rate constants are: kfwd = 7.00 × 10-5 s-1 and krev = 2.00 × 10-5 L mol-1 s-1, what is the value of Kc under these conditions?
2B(g)
If the rate constants are: kfwd = 7.00 × 10-5 s-1 and krev = 2.00 × 10-5 L mol-1 s-1, what is the value of Kc under these conditions?
(Multiple Choice)
4.8/5  (35)
(35)
Hydrogen sulfide can be formed in the following reaction: H2(g) + 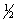 S2(g)
S2(g)  H2S(g) H°rxn = -92 kJ
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
H2S(g) H°rxn = -92 kJ
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
(Multiple Choice)
4.8/5  (44)
(44)
Consider the equilibrium reaction: N2O4(g)  2NO2(g) Which of the following correctly describes the relationship between Kc and Kp for the reaction?
2NO2(g) Which of the following correctly describes the relationship between Kc and Kp for the reaction?
(Multiple Choice)
4.7/5  (37)
(37)
The following reaction is at equilibrium at one atmosphere, in a closed container. NaOH(s) + CO2(g)  NaHCO3(s)
Which, if any, of the following actions will decrease the total amount of CO2 gas present at equilibrium?
NaHCO3(s)
Which, if any, of the following actions will decrease the total amount of CO2 gas present at equilibrium?
(Multiple Choice)
4.9/5  (40)
(40)
At a certain temperature the reaction CO2(g) + H2(g)  CO(g) + H2O(g)
Has Kc = 2.50. If 2.00 mol of carbon dioxide and 1.5 mol of hydrogen are placed in a 5.00 L vessel and equilibrium is established, what will be the concentration of carbon monoxide?
CO(g) + H2O(g)
Has Kc = 2.50. If 2.00 mol of carbon dioxide and 1.5 mol of hydrogen are placed in a 5.00 L vessel and equilibrium is established, what will be the concentration of carbon monoxide?
(Multiple Choice)
4.8/5  (41)
(41)
A mixture 0.500 mole of carbon monoxide and 0.400 mole of bromine was placed into a rigid 1.00-L container and the system was allowed to come to equilibrium. The equilibrium concentration of COBr2 was 0.233 M. What is the value of Kc for this reaction? CO(g) + Br2(g)  COBr2(g)
COBr2(g)
(Multiple Choice)
4.8/5  (32)
(32)
Nitrogen dioxide can dissociate to nitric oxide and oxygen. 2NO2(g)  2NO(g) + O2(g) H°rxn = +114 kJ
Under which reaction conditions would you expect to produce the largest amount of oxygen?
2NO(g) + O2(g) H°rxn = +114 kJ
Under which reaction conditions would you expect to produce the largest amount of oxygen?
(Multiple Choice)
4.8/5  (36)
(36)
Write the mass-action expression, Qc, for the following chemical reaction. NO(g) + 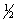 Br2(g)
Br2(g)  NOBr(g)
NOBr(g)
(Multiple Choice)
4.8/5  (26)
(26)
Consider the following gas-phase equilibrium reaction:
N2(g) + O2(g)  2NO(g) Kc = 4.10 × 10-4 at 2000°C
If 1.0 mol of NO is introduced into a 1.0 L container at 2000°C, what is the concentration of NO when equilibrium is reached?
2NO(g) Kc = 4.10 × 10-4 at 2000°C
If 1.0 mol of NO is introduced into a 1.0 L container at 2000°C, what is the concentration of NO when equilibrium is reached?
(Essay)
4.9/5  (40)
(40)
A chemical reaction has an equilibrium constant of 2 × 106. If this reaction is at equilibrium, select the one correct conclusion that can be made about the reaction.
(Multiple Choice)
4.8/5  (42)
(42)
The equilibrium constant Kc for the reaction A(g) + B(g)  C(g)
Is 0.76 at 150°C. If 0.800 mol of A is added to 0.600 mol of B in a 1.00-L container at 150°C, what will be the equilibrium concentration of C?
C(g)
Is 0.76 at 150°C. If 0.800 mol of A is added to 0.600 mol of B in a 1.00-L container at 150°C, what will be the equilibrium concentration of C?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 81 - 100 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)