Exam 16: Temperature and Heat
Exam 1: Units, Physical Quantities, and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum, Impulse, and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Equilibrium and Elasticity50 Questions
Exam 11: Fluid Mechanics50 Questions
Exam 12: Gravitation50 Questions
Exam 13: Periodic Motion50 Questions
Exam 14: Mechanical Waves44 Questions
Exam 15: Sound and Hearing66 Questions
Exam 16: Temperature and Heat63 Questions
Exam 17: Thermal Properties of Matter58 Questions
Exam 18: The First Law of Thermodynamics52 Questions
Exam 19: The Second Law of Thermodynamics50 Questions
Exam 20: Electric Charge and Electric Field58 Questions
Exam 21: Gausss Law41 Questions
Exam 22: Electric Potential55 Questions
Exam 23: Capacitance and Dielectrics52 Questions
Exam 24: Current, Resistance, and Electromotive Force50 Questions
Exam 25: Direct-Current Circuits53 Questions
Exam 26: Magnetic Field and Magnetic Forces36 Questions
Exam 27: Sources of Magnetic Field51 Questions
Exam 28: Electromagnetic Induction39 Questions
Exam 29: Inductance26 Questions
Exam 30: Alternating Current49 Questions
Exam 31: Electromagnetic Waves47 Questions
Exam 32: The Nature and Propagation of Light28 Questions
Exam 33: Geometric Optics81 Questions
Exam 34: Interference33 Questions
Exam 35: Diffraction49 Questions
Exam 36: Relativity51 Questions
Exam 37: Photons: Light Waves Behaving As Particles38 Questions
Exam 38: Particles Behaving As Waves52 Questions
Exam 39: Quantum Mechanics40 Questions
Exam 40: Atomic Structure41 Questions
Exam 41: Molecules and Condensed Matter31 Questions
Exam 42: Nuclear Physics89 Questions
Exam 43: Particle Physics and Cosmology44 Questions
Select questions type
A substance has a melting point of 20°C and a heat of fusion of 3.9 x 104 J/kg. The boiling point is 150°C and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K), 1000 J/(kg∙K), and 400 J/(kg∙K), respectively. The quantity of heat required to raise the temperature of 3.80 kg of the substance from -6°C to 128°C at a pressure of 1.0 atm, is closest to
(Multiple Choice)
4.7/5  (34)
(34)
A solid concrete wall 4.0 m by 2.4 m and 30 cm thick, with a thermal conductivity of 1.3 W/(m∙K), separates a basement at 18°C from the ground outside at 6°C. Under steady state conditions, how much heat flows through the wall in one hour?
(Multiple Choice)
4.9/5  (32)
(32)
A heat conducting rod, 0.90 m long, is made of an aluminum section that is 0.10 m long, and a copper section that is 0.80 m long. Both sections have cross-sectional areas of 0.00040 m2. The aluminum end is maintained at a temperature of 40°C and the copper end is at 150°C. The thermal conductivity of aluminum is 205 W/m∙K and of copper is 385 W/m∙K. Steady state has been reached, and no heat is lost through the well-insulated sides of the rod. The temperature of the aluminum-copper junction in the rod is closest to
(Multiple Choice)
5.0/5  (36)
(36)
Heat is added to a 2.0 kg piece of ice at a rate of 793.0 kW. How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
(Multiple Choice)
4.9/5  (33)
(33)
An architect is interested in estimating the heat loss (in kcal/s) through a sheet of insulating material as a function of the thickness of the sheet. Assuming fixed temperatures on the two faces of the sheet, which one of the graphs in the figure best represents the rate of heat transfer as a function of the thickness of the insulating sheet? 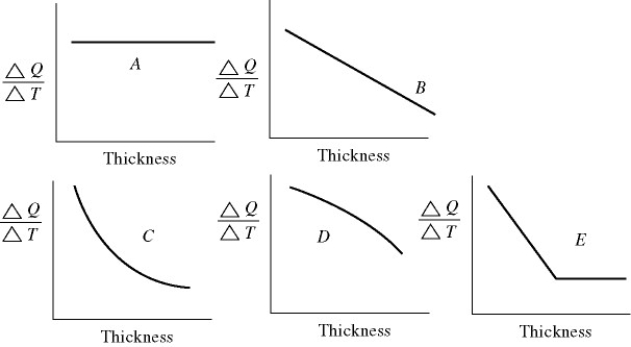
(Multiple Choice)
4.9/5  (38)
(38)
A solid metal sphere is 15.0 cm in diameter and has surface of uniform color. When its surface is at 112°C, you measure that it radiates energy at a rate of 71.3 W. What is the emissivity of the surface of this object? Any heat that enters the sphere from the outside environment is negligible. The Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.
(Short Answer)
4.8/5  (38)
(38)
The coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1. A 1.00-L glass beaker is filled to the brim with olive oil at room temperature. The beaker is placed on a range and the temperature of the oil and beaker increases by 25 C°. As a result, 0.0167 L of olive oil spill over the top of the beaker. What is the coefficient of linear expansion of the glass?
(Multiple Choice)
4.8/5  (31)
(31)
A cube at 100°C radiates heat at a rate of 80.0 J/s. If its surface temperature is increased to 200°C, the rate at which it will now radiate is closest to
(Multiple Choice)
4.8/5  (42)
(42)
A person makes ice tea by adding ice to 1.8 kg of hot tea, initially at 80°C. How many kilograms of ice, initially at 0.00°C, are required to bring the mixture to 10°C? The heat of fusion of ice is 334 kJ/kg, and we can assume that tea has essentially the same thermal properties as water, so its specific heat is 4190 J/(kg∙K).
(Multiple Choice)
4.9/5  (36)
(36)
It is necessary to determine the specific heat of an unknown object. The mass of the object is measured to be 199.0 g. It is determined experimentally that it takes 16.0 J to raise the temperature 10.0°C. Find the specific heat of the object.
(Multiple Choice)
4.9/5  (33)
(33)
If 2.0 g of water at 0.00°C is to be vaporized, how much heat must be added to it? The specific heat of water is 1.0 cal/g∙K, its heat of fusion is 80 cal/g, and its heat of vaporization is 539 cal/g.
(Multiple Choice)
4.9/5  (37)
(37)
Two experimental runs are performed to determine the calorimetric properties of an alcohol that has a melting point of -10°C. In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5.0°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C. The specific heat of water is 4190 J/kg·K. Assume that no heat is exchanged with the styrofoam container and with the surroundings. The heat of fusion of the alcohol is closest to
(Multiple Choice)
4.8/5  (41)
(41)
The coefficient of linear expansion of aluminum is 24 × 10-6 K-1 and the coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1. A novice cook, in preparation for deep-frying some potatoes, fills a 1.00-L aluminum pot to the brim and heats the oil and the pot from an initial temperature of 15°C to 190°C. To his consternation some olive oil spills over the top. How much?
(Multiple Choice)
4.9/5  (34)
(34)
Two identical concrete slabs lie flat and in contact with each other as shown in the figure. If the temperature increases by 40°C, the lower edges opposite the contact edges remained fixed in position, and the lower edges of the contact side remain in contact, at what angle will the slabs be tilted? The coefficient of thermal expansion of the concrete is 10 × 10-6/K. 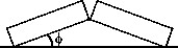
(Multiple Choice)
4.9/5  (42)
(42)
If you add 700 kJ of heat to 700 g of water at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 2.26 × 106 J/kg and its specific heat is 4190 J/(kg∙K).
(Multiple Choice)
4.9/5  (30)
(30)
A copper cylinder with a mass of 125 g and temperature of 345°C is cooled by dropping it into a glass beaker containing 565 g of water initially at 20.0°C. The mass of the beaker is 50.0 g and the specific heat of the glass is 840 J/kg∙K. What is the final equilibrium temperature of the system, assuming the cooling takes place very quickly, so that no energy is lost to the air? The specific heat of copper is 385 J/kg∙K and that of water is 4190 J/kg∙K.
(Short Answer)
4.8/5  (42)
(42)
A chunk of ice (T = -20°C) is added to a thermally insulated container of cold water (T = 0°C). What happens in the container?
(Multiple Choice)
4.9/5  (34)
(34)
A cube at 100.0°C radiates heat at a rate of 80.0 J/s. If the length of each side is cut in half, the rate at which it will now radiate is closest to
(Multiple Choice)
4.9/5  (38)
(38)
The walls of an ice chest are made of 2.00-mm-thick insulation having a thermal conductivity 0.00300 W/m∙K. The total surface area of the ice chest is 1.20 m2. If 4.00 kg of ice at 0.00°C are placed in the chest and the temperature of the outside surface of the chest is 20.0°C, how long does it take the ice to melt under steady state conditions? The latent heat of fusion of water is 79.6 cal/g = 334 kJ/kg.
(Multiple Choice)
4.8/5  (32)
(32)
Showing 21 - 40 of 63
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)