Exam 16: Temperature and Heat
Exam 1: Units, Physical Quantities, and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum, Impulse, and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Equilibrium and Elasticity50 Questions
Exam 11: Fluid Mechanics50 Questions
Exam 12: Gravitation50 Questions
Exam 13: Periodic Motion50 Questions
Exam 14: Mechanical Waves44 Questions
Exam 15: Sound and Hearing66 Questions
Exam 16: Temperature and Heat63 Questions
Exam 17: Thermal Properties of Matter58 Questions
Exam 18: The First Law of Thermodynamics52 Questions
Exam 19: The Second Law of Thermodynamics50 Questions
Exam 20: Electric Charge and Electric Field58 Questions
Exam 21: Gausss Law41 Questions
Exam 22: Electric Potential55 Questions
Exam 23: Capacitance and Dielectrics52 Questions
Exam 24: Current, Resistance, and Electromotive Force50 Questions
Exam 25: Direct-Current Circuits53 Questions
Exam 26: Magnetic Field and Magnetic Forces36 Questions
Exam 27: Sources of Magnetic Field51 Questions
Exam 28: Electromagnetic Induction39 Questions
Exam 29: Inductance26 Questions
Exam 30: Alternating Current49 Questions
Exam 31: Electromagnetic Waves47 Questions
Exam 32: The Nature and Propagation of Light28 Questions
Exam 33: Geometric Optics81 Questions
Exam 34: Interference33 Questions
Exam 35: Diffraction49 Questions
Exam 36: Relativity51 Questions
Exam 37: Photons: Light Waves Behaving As Particles38 Questions
Exam 38: Particles Behaving As Waves52 Questions
Exam 39: Quantum Mechanics40 Questions
Exam 40: Atomic Structure41 Questions
Exam 41: Molecules and Condensed Matter31 Questions
Exam 42: Nuclear Physics89 Questions
Exam 43: Particle Physics and Cosmology44 Questions
Select questions type
An 80-g aluminum calorimeter contains 380 g of water at an equilibrium temperature of 20°C. A 120-g piece of metal, initially at 352°C, is added to the calorimeter. The final temperature at equilibrium is 32°C. Assume there is no external heat exchange. The specific heats of aluminum and water are 910 J/kg·K and 4190 J/kg·K, respectively. The specific heat of the metal is closest to
(Multiple Choice)
4.8/5  (40)
(40)
A 648-g empty iron kettle is put on a stove. How much heat, in joules, must it absorb to raise its temperature from 15.0°C to 37.0°C? (The specific heat for iron is 113 cal/kg•C°, 1 cal = 4.190 J)
(Multiple Choice)
4.8/5  (42)
(42)
What is the steady state rate of heat flow through a pane of glass that is 40.0 cm by 30.0 cm with a thickness of 4.00 mm when the outside temperature of the glass is -10.0°C and its inside temperature is 25.0°C? The thermal conductivity of glass is 0.105 W/(m∙K), the specific heat of glass is 0.180 cal/(g∙°C), and 1 cal = 4.190 J.
(Multiple Choice)
4.7/5  (33)
(33)
It is a well-known fact that water has a higher specific heat than iron. Now, consider equal masses of water and iron that are initially in thermal equilibrium. The same amount of heat, 30 calories, is added to each one. Which statement is true?
(Multiple Choice)
5.0/5  (46)
(46)
A glass flask has a volume of 500 mL at a temperature of 20°C. The flask contains 492 mL of mercury at 20°C. The temperature of the mercury and flask is raised until the mercury reaches the 500 mL reference mark. The coefficients of volume expansion of mercury and glass are 18 × 10-5 K-1 and 2.0 × 10-5 K-1, respectively. The temperature at which this occurs is closest to
(Multiple Choice)
4.9/5  (38)
(38)
If we use 67 W of power to heat 148 g of water, how long will it take to raise the temperature of the water from 15°C to 25°C? The specific heat of water is 4190 J/kg•K.
(Multiple Choice)
4.9/5  (42)
(42)
Two metal rods, one silver and the other copper, are both attached to a steam chamber as shown in the figure, with a temperature of 100°C, at one end, and an ice water bath, with a temperature of 0°C, at the other. The rods are 5.0 cm long and have a square cross-section, 2.0 cm on a side. When steady state has been reached, how much heat flows through the two rods in 1.0 min? The thermal conductivity of silver is 417 W/(m∙K), and that of copper is 395 W/(m∙K). No heat is exchanged between the rods and the surroundings, except at their ends. 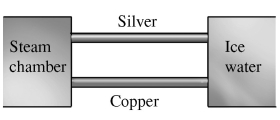
(Multiple Choice)
4.9/5  (33)
(33)
A rod, with sides insulated to prevent heat loss, has one end immersed in boiling water 100°C and the other end in a water-ice mixture at 0.00°C. The rod has uniform cross-sectional area of 4.04 cm2 and length of 91 cm. Under steady state conditions, the heat conducted by the rod melts the ice at a rate of 1.0 g every 34 seconds. What is the thermal conductivity of the rod? (The heat of fusion of water is 3.34 × 105 J/kg.)
(Short Answer)
4.8/5  (36)
(36)
A substance has a melting point of 20°C and a heat of fusion of 3.5 × 104 J/kg. The boiling point is 150°C and the heat of vaporization is 7.0 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K), 1000 J/(kg∙K), and 400 J/(kg∙K), respectively. The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C, at a pressure of 1.0 atmosphere, is closest to
(Multiple Choice)
4.7/5  (43)
(43)
How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 87°C to produce water at a final temperature of 10.0°C? Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg·C° and of ice is 2050 J/kg·C°. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
(Short Answer)
4.8/5  (35)
(35)
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper; the aluminum and the copper are in thermal contact. The specific heat of aluminum is more than double that of copper. Which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?
(Multiple Choice)
4.8/5  (37)
(37)
A heat conducting rod, 1.40 m long, is made of an aluminum section that is 0.50 m long and a copper section that is 0.90 m long. Both sections have cross-sectional areas of 0.00040 m2. The aluminum end and the copper end are maintained at temperatures of 40°C and 280°C, respectively. The thermal conductivity of aluminum is 205 W/m∙K of copper is 385 W/m∙K. The rate at which heat is conducted in the rod is closest to
(Multiple Choice)
4.8/5  (34)
(34)
You want to insert an aluminum rod, which at 20°C has a radius of 1.000200 cm into a copper tube which has a radius of 1.000100 cm at the same temperature. You decide to put both of them in the refrigerator. At what temperature will the rod just fit if both are cooled to the same temperature? The coefficient of thermal expansion for aluminum is 2.4 × 10-5 K-1, and that of copper is 1.7 × 10-5 K-1.
(Multiple Choice)
4.7/5  (37)
(37)
A rod has a length 2.00000 m at 10.0°C. The length of the rod increases to 2.00060 m when the temperature increases to 30.0°C. What is the coefficient of linear expansion of the material from which the rod is made?
(Multiple Choice)
4.9/5  (37)
(37)
Under steady state conditions, a piece of wood 350 mm by 350 mm and 15 mm thick conducts heat through its thickness and loses no appreciable heat through its well-insulated sides. The rate of heat flow is measured to be 14.0 W when the temperature difference across its thickness is 28 C°. Determine the thermal conductivity of this wood.
(Multiple Choice)
4.9/5  (30)
(30)
A spherical object 25.0 cm in diameter having an emissivity of 0.800 is held at a temperature of 275°C by an internal heater. This object is embedded in a very large vat of water at 100.0°C and atmospheric pressure. At what maximum rate (in g/min) is the water evaporating in the vat due to the radiated heat it receives from the object? You can ignore any heat radiated by the water. The latent heat of fusion for water is 33,400 J/kg, its latent heat of vaporization is 2.26 × 106 J/kg, and the Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.
(Short Answer)
4.8/5  (29)
(29)
A 200-g metal container, insulated on the outside, holds 100 g of water in thermal equilibrium at 22.00°C. A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
(Multiple Choice)
4.7/5  (30)
(30)
What is the net power that a person loses through radiation if her surface area is 1.20 m2, if her emissivity is 0.895, if her skin temperature is 300 K, and if she is in a room that is at a temperature of 17°C? The Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.
(Multiple Choice)
4.9/5  (44)
(44)
1.000 L of water at 20.00°C will occupy what volume if it is heated to 80.00°C? Water has a volume expansion coefficient of 210 × 10-6/C°. (Express your answer to 4 significant figures.)
(Multiple Choice)
4.9/5  (43)
(43)
(a) Internal human body temperature is often stated to be normal at 98.6°F. What is this temperature on the Celsius and Kelvin scales?
(b) Gallium boils at 2205°C. What is the corresponding temperature in the Fahrenheit and Kelvin scales?
(c) The boiling point of liquid nitrogen is 77.0 K. What is the corresponding temperature in the Fahrenheit and Celsius scales?
(Short Answer)
4.9/5  (39)
(39)
Showing 41 - 60 of 63
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)