Exam 7: Structure and Synthesis of Alkenes; Elimination
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Cyclohexane has a molecular formula of C6H12 and an unsaturation number of 1. Is this compound considered to be unsaturated? Why or why not?
(Essay)
4.9/5  (31)
(31)
Which of the following alkenes has the smallest molar heat of hydrogenation (ie, releases the least heat upon hydrogenation)?
(Multiple Choice)
4.8/5  (35)
(35)
A newly isolated natural product was found to have the molecular formula C15H28O2. By hydrogenating a sample of the compound, it was determined to possess one π bond. How many rings are present in the compound?
(Multiple Choice)
5.0/5  (32)
(32)
Which of the alkyl chlorides listed below undergoes dehydrohalogenation in the presence of a strong base to give pent-2-ene as the only alkene product?
(Multiple Choice)
4.8/5  (44)
(44)
Provide the proper IUPAC name for the alkene shown below.
CH2
 CHCH2CH2CH2CH3
CHCH2CH2CH2CH3
(Short Answer)
4.8/5  (39)
(39)
Carbon-carbon single bonds tend to be ________ and ________ than carbon-carbon double bonds.
(Multiple Choice)
4.9/5  (37)
(37)
Which sequence ranks the following compounds in order of increasing heat of hydrogenation, ΔHhyd?
1 cis-2-butene 2 1-butene 3 cyclohexene
(Multiple Choice)
4.8/5  (30)
(30)
Give the structures of three examples of molecules that have a molecular formula of C6H8. One of the structures should have only one ring and one of the structures should have no rings.
(Essay)
4.8/5  (44)
(44)
The carbon-carbon bond length in ethylene is ________ than the carbon-carbon bond length in ethane, and the HCH bond angle in ethylene is ________ the HCH bond angle in ethane.
(Multiple Choice)
4.9/5  (32)
(32)
Describe the major elimination products of the following reaction, including any stereochemistry, and indicate which structure will predominate. Explain and defend your answer. 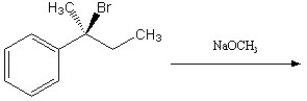
(Essay)
4.8/5  (29)
(29)
The dehydrogenation of butane to trans-but-2-ene has ∆H° = +116 kJ/mol (+27.6 kcal/mol) and ∆S° = +117 J-kelvin/mol (+28.0 cal-kelvin/mol). Compute the value of ∆G° for dehydrogenation at room temperature (25°C or 298°K). Is dehydrogenation favored or disfavored?
(Essay)
4.8/5  (36)
(36)
Draw the alkene of formula C5H10 which evolves the most heat per mole upon hydrogenation.
(Essay)
4.9/5  (31)
(31)
The steroid testosterone has the molecular formula C19H28O2. Given that there are two π bonds in a molecule of testosterone, how many rings are present in each molecule?
(Multiple Choice)
4.8/5  (34)
(34)
Provide the reagents necessary for carrying out the transformation of cyclopentane to cyclopentene.
(Short Answer)
4.9/5  (40)
(40)
Draw a mechanism and use a chair structure to explain the major product that is formed in the reaction below. 
(Essay)
4.8/5  (34)
(34)
Showing 21 - 40 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)