Exam 7: Structure and Synthesis of Alkenes; Elimination
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Dehydrohalogenation of an alkyl halide by treating it with a strong base to yield an alkene product typically occurs by what reaction mechanism?
(Multiple Choice)
4.9/5  (33)
(33)
Draw all likely products of the following reaction and circle the product you expect to predominate.
CH3CH(OH)CH2CH2CH3 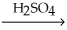
(Essay)
4.9/5  (37)
(37)
Provide the structure of the major organic product of the reaction below. 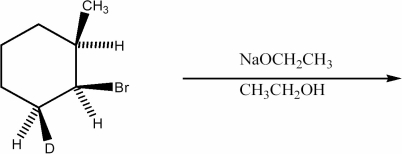
(Essay)
4.8/5  (39)
(39)
Draw all likely alkene products in the following reaction and circle the product you expect to predominate. 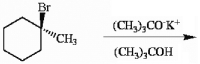
(Essay)
4.9/5  (26)
(26)
Consider the constitutional isomers 2-methylbut-1-ene, 2-methylbut-2-ene, and 3-methylbut-1-ene. When each of these alkenes is subjected to catalytic hydrogenation (H2, Pt), a single product results. Which of the following best describes the structural relationship among these products?
(Multiple Choice)
4.9/5  (39)
(39)
Which has the smaller heat of hydrogenation, (E)-2-pentene or (Z)-2-pentene? What is the structural origin of this difference?
(Essay)
4.7/5  (30)
(30)
Why is rotation about the carbon-carbon double bond in alkenes prohibited while relatively free rotation can occur about the carbon-carbon single bond in alkanes?
(Essay)
4.9/5  (30)
(30)
Using Zaitsev's rule, choose the most stable alkene among the following.
(Multiple Choice)
4.7/5  (40)
(40)
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 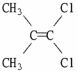
(Multiple Choice)
4.8/5  (35)
(35)
3-Methylpentane can be dehydrogenated to form an alkene with molecular formula of C6H12. What is the most stable alkene that could be formed? Name it with the appropriate stereochemical designator.
(Short Answer)
4.9/5  (33)
(33)
The trans isomers of cycloalkenes with rings containing fewer than ________ atoms are unstable at room temperature.
(Short Answer)
4.8/5  (37)
(37)
A chemist has isolated a new natural product and determined its molecular formula to be C24H40O4. In hydrogenation experiments the chemist found that each mole of the natural product reacted with two moles of H2. How many rings are present in the structure of the new natural product?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 101 - 120 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)