Exam 7: Structure and Synthesis of Alkenes; Elimination
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
In the group shown below, which of the following alcohols is (are) likely to yield a product where skeletal rearrangement has occurred when treated with sulfuric acid?
3-methyl-3-pentanol, 3,3-dimethyl-2-butanol, 2,2-dimethylcyclohexanol
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following statements best describes the relative bond dissociation energies of the sigma and pi bonds present in the carbon-carbon double bond of an alkene?
(Multiple Choice)
4.8/5  (37)
(37)
Draw all likely products of the following reaction and circle the product you expect to predominate. 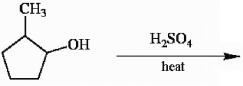
(Essay)
4.8/5  (39)
(39)
Which is the more stable diene shown below? Briefly explain your answer. 
(Essay)
4.9/5  (34)
(34)
Describe the major products of the following reaction and predict which of the products would predominate. Explain and defend your answer. 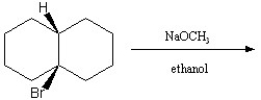
(Essay)
4.8/5  (40)
(40)
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 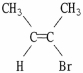
(Multiple Choice)
4.8/5  (49)
(49)
Dehydration of an unknown alcohol with concentrated H2SO4 results in the formation of all of the following alkene products. What is/are the possible structures of the original alcohol? 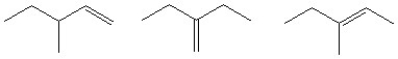
(Multiple Choice)
4.8/5  (36)
(36)
The prostaglandin precursor arachidonic acid has the molecular formula C20H32O2. Given that arachidonic acid is an acyclic carboxylic acid that contains no carbon-carbon triple bonds, how many carbon-carbon double bonds are present?
(Multiple Choice)
4.8/5  (32)
(32)
Provide the structure of the major organic product of the reaction below. 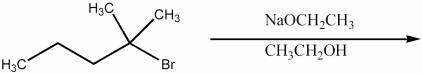
(Essay)
4.7/5  (29)
(29)
Which of the following best approximates the CCC bond angle of propene?
(Multiple Choice)
4.9/5  (32)
(32)
Dehydrohalogenation of 2-bromobutane in the presence of a strong base proceeds via which of the following mechanistic pathways?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following best describes the geometry about the carbon-carbon double bond in the alkene below? 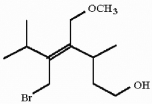
(Multiple Choice)
4.8/5  (33)
(33)
Circle the alkene below which has the smallest heat of hydrogenation. 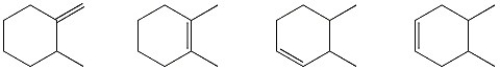
(Essay)
4.7/5  (33)
(33)
The industrial method of making alkenes is via catalytic cracking. If decane is subjected to these conditions, hexane and 1-butene are one set of possible products. Draw the products in line-angle structures.
(Essay)
4.9/5  (40)
(40)
Using Zaitsev's rule, choose the most stable alkene among the following.
(Multiple Choice)
4.8/5  (43)
(43)
There are three isomeric methylbutene structures. Draw each of them and then circle the isomer with the highest heat of hydrogenation.
(Essay)
4.8/5  (37)
(37)
Showing 81 - 100 of 131
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)