Exam 2: The Chemical Context of Life
Exam 1: Evolution, the Themes of Biology, and Scientific Inquiry51 Questions
Exam 2: The Chemical Context of Life61 Questions
Exam 3: Water and Life55 Questions
Exam 4: Carbon and the Molecular Diversity of Life58 Questions
Exam 5: The Structure and Function of Large Biological Molecules70 Questions
Exam 6: A Tour of the Cell66 Questions
Exam 7: Membrane Structure and Function68 Questions
Exam 8: An Introduction to Metabolism67 Questions
Exam 9: Cellular Respiration and Fermentation68 Questions
Exam 10: Photosynthesis65 Questions
Exam 11: Cell Communication65 Questions
Exam 12: The Cell Cycle66 Questions
Exam 13: Meiosis and Sexual Life Cycles64 Questions
Exam 14: Mendel and the Gene Idea62 Questions
Exam 15: The Chromosomal Basis of Inheritance58 Questions
Exam 16: The Molecular Basis of Inheritance65 Questions
Exam 17: Gene Expression: From Gene to Protein67 Questions
Exam 18: Regulation of Gene Expression66 Questions
Exam 19: Viruses54 Questions
Exam 20: DNA Tools and Biotechnology57 Questions
Exam 21: Genomes and Their Evolution44 Questions
Exam 22: Descent with Modification: A Darwinian View of Life60 Questions
Exam 23: The Evolution of Populations64 Questions
Exam 24: The Origin of Species67 Questions
Exam 25: The History of Life on Earth59 Questions
Exam 26: Phylogeny and the Tree of Life75 Questions
Exam 27: Bacteria and Archaea75 Questions
Exam 28: Protists79 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land82 Questions
Exam 30: Plant Diversity II: The Evolution of Seed Plants80 Questions
Exam 31: Fungi75 Questions
Exam 32: An Overview of Animal Diversity67 Questions
Exam 33: An Introduction to Invertebrates83 Questions
Exam 34: The Origin and Evolution of Vertebrates82 Questions
Exam 35: Vascular Plant Structure, Growth, and Development65 Questions
Exam 36: Resource Acquisition and Transport in Vascular Plants74 Questions
Exam 37: Soil and Plant Nutrition52 Questions
Exam 38: Angiosperm Reproduction and Biotechnology60 Questions
Exam 39: Plant Responses to Internal and External Signals61 Questions
Exam 40: Basic Principles of Animal Form and Function68 Questions
Exam 41: Animal Nutrition64 Questions
Exam 42: Circulation and Gas Exchange67 Questions
Exam 43: The Immune System69 Questions
Exam 44: Osmoregulation and Excretion64 Questions
Exam 45: Hormones and the Endocrine System66 Questions
Exam 46: Animal Reproduction68 Questions
Exam 47: Animal Development70 Questions
Exam 48: Neurons, Synapses, and Signaling68 Questions
Exam 49: Nervous Systems65 Questions
Exam 50: Sensory and Motor Mechanisms67 Questions
Exam 51: Animal Behavior69 Questions
Exam 52: An Introduction to Ecology and the Biosphere68 Questions
Exam 53: Population Ecology69 Questions
Exam 54: Community Ecology71 Questions
Exam 55: Ecosystems and Restoration Ecology68 Questions
Exam 56: Conservation Biology and Global Change69 Questions
Select questions type
What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms?
(Multiple Choice)
4.8/5  (41)
(41)
The atomic number of nitrogen is 7. Nitrogen-15 has a greater mass number than nitrogen-14 because the atomic nucleus of nitrogen-15 contains ________.
(Multiple Choice)
4.9/5  (30)
(30)
We can represent atoms by listing the number of protons, neutrons, and electrons-for example, 2p⁺, 2n⁰, 2e⁻ for helium. Which of the following represents the ¹⁸O isotope of oxygen?
(Multiple Choice)
4.9/5  (34)
(34)
The left to right order of elements in the periodic table is based on their ________.
(Multiple Choice)
4.7/5  (34)
(34)
Bonds between two atoms that are equally electronegative are ________.
(Multiple Choice)
4.8/5  (42)
(42)
Refer to the following figure to answer the questions below.
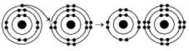 -How many electrons are involved in a triple covalent bond?
-How many electrons are involved in a triple covalent bond?
(Multiple Choice)
4.9/5  (34)
(34)
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
(Multiple Choice)
4.7/5  (36)
(36)
You are asked to indicate the type and number of atoms in a molecule. Which representation would work best?
(Multiple Choice)
4.8/5  (35)
(35)
Refer to the following figure to answer the questions below.
3H₂ + N₂  2NH₃
-Which of the following factors will increase the rate of reaction in the forward direction?
2NH₃
-Which of the following factors will increase the rate of reaction in the forward direction?
(Multiple Choice)
4.8/5  (28)
(28)
Refer to the following figure to answer the questions below.
3H₂ + N₂  2NH₃
-Which of the following correctly describes chemical equilibrium?
2NH₃
-Which of the following correctly describes chemical equilibrium?
(Multiple Choice)
4.9/5  (39)
(39)
Refer to the following figure to answer the questions below.
 -Elements found on the left side of the periodic table contain outer shells that are ________; these elements tend to form ________ in solution.
-Elements found on the left side of the periodic table contain outer shells that are ________; these elements tend to form ________ in solution.
(Multiple Choice)
4.9/5  (38)
(38)
What is the maximum number of covalent bonds that an oxygen atom with atomic number 8 can make with hydrogen?
(Multiple Choice)
4.9/5  (36)
(36)
Nitrogen (N) is more electronegative than hydrogen (H). Which of the following is a correct statement about the atoms in ammonia (NH₃)?
(Multiple Choice)
4.8/5  (36)
(36)
A salamander relies on hydrogen bonding to stick to various surfaces. Therefore, a salamander would have the greatest difficulty clinging to a ________.
(Multiple Choice)
4.8/5  (32)
(32)
In a chemical reaction, the element ¹³Al will most preferably ________.
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 61
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)