Exam 8: An Introduction to Metabolism
Exam 1: Evolution, the Themes of Biology, and Scientific Inquiry51 Questions
Exam 2: The Chemical Context of Life61 Questions
Exam 3: Water and Life55 Questions
Exam 4: Carbon and the Molecular Diversity of Life58 Questions
Exam 5: The Structure and Function of Large Biological Molecules70 Questions
Exam 6: A Tour of the Cell66 Questions
Exam 7: Membrane Structure and Function68 Questions
Exam 8: An Introduction to Metabolism67 Questions
Exam 9: Cellular Respiration and Fermentation68 Questions
Exam 10: Photosynthesis65 Questions
Exam 11: Cell Communication65 Questions
Exam 12: The Cell Cycle66 Questions
Exam 13: Meiosis and Sexual Life Cycles64 Questions
Exam 14: Mendel and the Gene Idea62 Questions
Exam 15: The Chromosomal Basis of Inheritance58 Questions
Exam 16: The Molecular Basis of Inheritance65 Questions
Exam 17: Gene Expression: From Gene to Protein67 Questions
Exam 18: Regulation of Gene Expression66 Questions
Exam 19: Viruses54 Questions
Exam 20: DNA Tools and Biotechnology57 Questions
Exam 21: Genomes and Their Evolution44 Questions
Exam 22: Descent with Modification: A Darwinian View of Life60 Questions
Exam 23: The Evolution of Populations64 Questions
Exam 24: The Origin of Species67 Questions
Exam 25: The History of Life on Earth59 Questions
Exam 26: Phylogeny and the Tree of Life75 Questions
Exam 27: Bacteria and Archaea75 Questions
Exam 28: Protists79 Questions
Exam 29: Plant Diversity I: How Plants Colonized Land82 Questions
Exam 30: Plant Diversity II: The Evolution of Seed Plants80 Questions
Exam 31: Fungi75 Questions
Exam 32: An Overview of Animal Diversity67 Questions
Exam 33: An Introduction to Invertebrates83 Questions
Exam 34: The Origin and Evolution of Vertebrates82 Questions
Exam 35: Vascular Plant Structure, Growth, and Development65 Questions
Exam 36: Resource Acquisition and Transport in Vascular Plants74 Questions
Exam 37: Soil and Plant Nutrition52 Questions
Exam 38: Angiosperm Reproduction and Biotechnology60 Questions
Exam 39: Plant Responses to Internal and External Signals61 Questions
Exam 40: Basic Principles of Animal Form and Function68 Questions
Exam 41: Animal Nutrition64 Questions
Exam 42: Circulation and Gas Exchange67 Questions
Exam 43: The Immune System69 Questions
Exam 44: Osmoregulation and Excretion64 Questions
Exam 45: Hormones and the Endocrine System66 Questions
Exam 46: Animal Reproduction68 Questions
Exam 47: Animal Development70 Questions
Exam 48: Neurons, Synapses, and Signaling68 Questions
Exam 49: Nervous Systems65 Questions
Exam 50: Sensory and Motor Mechanisms67 Questions
Exam 51: Animal Behavior69 Questions
Exam 52: An Introduction to Ecology and the Biosphere68 Questions
Exam 53: Population Ecology69 Questions
Exam 54: Community Ecology71 Questions
Exam 55: Ecosystems and Restoration Ecology68 Questions
Exam 56: Conservation Biology and Global Change69 Questions
Select questions type
How does a noncompetitive inhibitor decrease the rate of an enzyme-catalyzed reaction?
(Multiple Choice)
4.9/5  (32)
(32)
Biological systems use free energy based on empirical data that all organisms require a constant energy input. The first law of thermodynamics states that energy can be neither created nor destroyed. For living organisms, which of the following statements is an important consequence of this first law?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following statements is consistent with the second law of thermodynamics?
(Multiple Choice)
4.8/5  (45)
(45)
Use the following information to answer the question below.
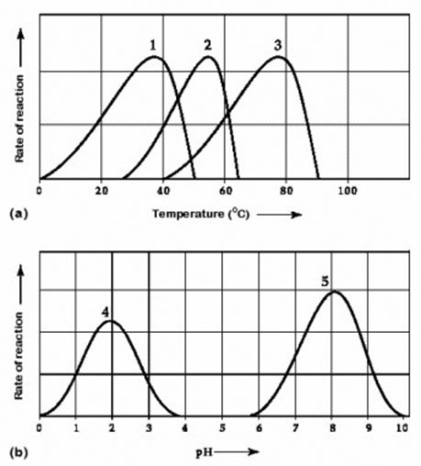 Activity of various enzymes at various temperatures (a) and at various pH (b)
Which temperature and pH profile curves on the graphs are most likely associated with an enzyme isolated from a human stomach where conditions are strongly acid?
Activity of various enzymes at various temperatures (a) and at various pH (b)
Which temperature and pH profile curves on the graphs are most likely associated with an enzyme isolated from a human stomach where conditions are strongly acid?
(Multiple Choice)
4.8/5  (36)
(36)
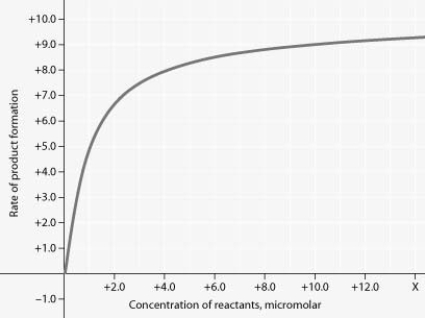 Rate of an enzyme-catalyzed reaction as a function of varying reactant concentration, with the concentration of enzyme constant.
For the enzyme-catalyzed reaction shown in the figure, if the initial reactant concentration is 1.0 micromolar, which of these treatments will cause the greatest increase in the rate of the reaction?
Rate of an enzyme-catalyzed reaction as a function of varying reactant concentration, with the concentration of enzyme constant.
For the enzyme-catalyzed reaction shown in the figure, if the initial reactant concentration is 1.0 micromolar, which of these treatments will cause the greatest increase in the rate of the reaction?
(Multiple Choice)
4.7/5  (31)
(31)
The mathematical expression for the change in free energy of a system is ΔG = ΔH - TΔS. Which of the following statements is correct?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements describes a key component of the induced fit hypothesis of enzyme catalysis?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 61 - 67 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)