Exam 4: Reactions in Aqueous Solution
Exam 1: Introduction to Chemistry96 Questions
Exam 2: Molecules, Ions, and Chemical Formulas110 Questions
Exam 3: Chemical Reactions88 Questions
Exam 4: Reactions in Aqueous Solution95 Questions
Exam 5: Energy Changes in Chemical Reactions93 Questions
Exam 6: The Structure of Atoms110 Questions
Exam 7: The Periodic Table and Periodic Trends97 Questions
Exam 8: Ionic Versus Covalent Bonding88 Questions
Exam 9: Molecular Geometry and Covalent Bonding Models89 Questions
Exam 10: Gases84 Questions
Exam 11: Liquids99 Questions
Exam 12: Solids97 Questions
Exam 13: Solutions96 Questions
Exam 14: Chemical Kinetics85 Questions
Exam 15: Chemical Equilibrium73 Questions
Exam 16: Aqueous Acidbase Equilibriums97 Questions
Exam 17: Solubility and Complexation Equilibriums85 Questions
Exam 18: Chemical Thermodynamics108 Questions
Exam 19: Electrochemistry95 Questions
Exam 20: Nuclear Chemistry95 Questions
Exam 21: Periodic Trends and the S-Block Elements93 Questions
Exam 22: The P-Block Elements94 Questions
Exam 23: The D-Block Elements95 Questions
Exam 24: Organic Compounds111 Questions
Select questions type
The insoluble product that forms in a precipitation reaction is called a(n) _____.
(Short Answer)
4.9/5  (42)
(42)
Individual cations and anions that are each surrounded by their own shell of water molecules are called _____.
(Short Answer)
4.9/5  (34)
(34)
Given: 2C6H5COOH + Na2CO3 2C6H5COONa + H2O + CO2 What mass of CO2 is formed when 275 mL of 0.150 M Na2CO3 is mixed with 775 mL of 0.254 M C6H5COOH?
(Multiple Choice)
4.9/5  (28)
(28)
The full molecular chemical equation shows all the substances present in the form in which they actually exist in the solution.
(True/False)
4.8/5  (42)
(42)
The scrubbers in coal-burning power plants use _____ to trap SO2.
(Multiple Choice)
4.8/5  (37)
(37)
Given complete ionic equation: 2Ag+(aq) + 2NO3−(aq) + 2K+(aq) + Cr2O72−(aq) Ag2Cr2O7(s) + 2K+(aq) + 2NO3−(aq) Convert the given equation into an overall chemical equation.
(Multiple Choice)
4.9/5  (24)
(24)
Why are precipitation reactions called double displacement reactions?
(Essay)
4.8/5  (32)
(32)
Given: CH3CH2COOH + H2O CH3CH2COO− + H3O+ What volume of 0.500 M CH3CH2COOH is reacting with water to form 2.86 g of CH3CH2COO−?
(Multiple Choice)
4.9/5  (36)
(36)
Given: 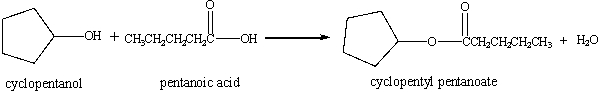 If 885 mL of 0.250 M pentanoic acid(molar mass = 102 g/mol) is mixed with 885 mL of 0.524 M cyclopentanol(molar mass = 86.1 g/mol), the 0.524 M cyclopentanol acts as the limiting agent.
If 885 mL of 0.250 M pentanoic acid(molar mass = 102 g/mol) is mixed with 885 mL of 0.524 M cyclopentanol(molar mass = 86.1 g/mol), the 0.524 M cyclopentanol acts as the limiting agent.
(True/False)
5.0/5  (41)
(41)
The ions that do not participate in the actual reaction are called _____.
(Short Answer)
4.8/5  (40)
(40)
In the equation given below, cyclopentanol (molar mass = 86.1 g/mol) reacts with pentanoic acid (molar mass = 102 g/mol) to produce cyclopentanoate(molar mass = 170.2 g/mol) and water. The volume of 0.297 M pentanoic acid needed for the complete reaction of 40.0 g cyclopentanol is _____ mL. 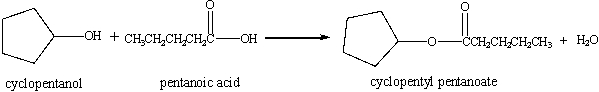
(Multiple Choice)
4.9/5  (35)
(35)
Showing 61 - 80 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)