Exam 4: Quantities of Reactants and Products
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 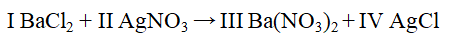
(Multiple Choice)
4.8/5  (38)
(38)
If 225 g of carbon reacts with excess sulfur dioxide to produce 195 g of carbon disulfide, what is the percent yield for the reaction? 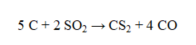
(Multiple Choice)
4.7/5  (36)
(36)
How many grams of Fe2O3 are formed by the complete reaction of 6.75 moles of iron? 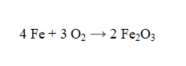
(Multiple Choice)
4.8/5  (41)
(41)
What is the maximum possible quantity of product obtained from a chemical reaction called?
(Multiple Choice)
4.8/5  (39)
(39)
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 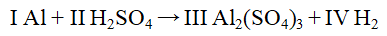
(Multiple Choice)
4.8/5  (42)
(42)
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients?
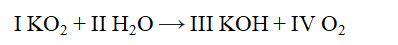
(Multiple Choice)
4.9/5  (35)
(35)
The complete reaction of 2.63 g of iron with 3.34 g of chlorine (Cl2) produces a compound with the formula FexCly. What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (26)
(26)
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 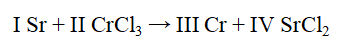
(Multiple Choice)
4.7/5  (39)
(39)
A decomposition reaction occurs when _____________ reactant(s) produces two or more products.
(Short Answer)
4.8/5  (35)
(35)
The Roman numerals in the equation given represent the coefficients in the balanced chemical equation. Give their values.
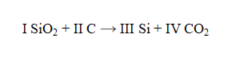
(Short Answer)
4.8/5  (42)
(42)
How many mol of ammonia will be formed from the complete reaction of 45.0 g of H2? 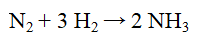
(Multiple Choice)
4.9/5  (34)
(34)
The complete reaction of 16.12 g of titanium with 23.88 g of chlorine (Cl2) produces a compound with the formula TixCly. What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (40)
(40)
How many grams of Al2O3 are formed by the complete reaction of 48.5 g of Fe2O3? 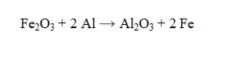
(Multiple Choice)
4.9/5  (43)
(43)
In the reaction given, if 25.0 g of each reactant is present, which reactant is in excess?
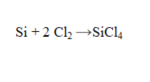
(Short Answer)
4.9/5  (34)
(34)
In the reaction given below, how many grams of C14H9Cl5 will be produced by the reaction of 25.0 g of each of the starting materials? 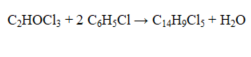
(Multiple Choice)
4.9/5  (42)
(42)
How many grams of HCl are required to completely react with 25.0 g of aluminum? 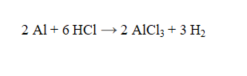
(Multiple Choice)
4.7/5  (31)
(31)
How many grams of PH3 are formed by the complete reaction of 52.5 g of H2? 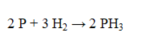
(Multiple Choice)
4.8/5  (32)
(32)
Showing 21 - 40 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)