Exam 4: Quantities of Reactants and Products
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
In the reaction given below, how many grams of sodium metal are consumed if 2.02 g of hydrogen gas are produced? 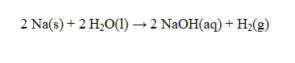
(Multiple Choice)
4.9/5  (35)
(35)
How many moles of H2O are formed from the complete combustion of 45.0 g of methanol, CH3OH? 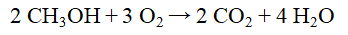
(Multiple Choice)
4.7/5  (36)
(36)
If 110.0 g of iron reacts with 64.0 g of oxygen, what is the theoretical yield of Fe2O3? 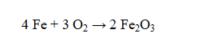
(Multiple Choice)
4.9/5  (41)
(41)
In the reaction given below, for every two molecules of aluminum oxide consumed, how many molecules of oxygen are produced? 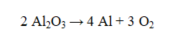
(Multiple Choice)
4.9/5  (34)
(34)
Showing 61 - 65 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)