Exam 4: Quantities of Reactants and Products
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
In the reaction shown below, which substances are dissolved in water? 
(Multiple Choice)
4.8/5  (39)
(39)
The efficiency of a particular synthesis method is evaluated by determining the:
(Multiple Choice)
4.8/5  (34)
(34)
If 32.0 g of oxygen reacts with sufficient magnesium to produce magnesium oxide, what is the theoretical yield? 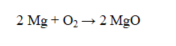
(Multiple Choice)
4.9/5  (28)
(28)
Which statement regarding the complete combustion of a carbon compound is correct?
(Multiple Choice)
4.8/5  (41)
(41)
The reaction below is an example of a(n) _____________ reaction.
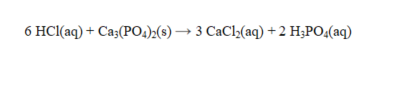
(Short Answer)
4.8/5  (39)
(39)
How many grams of Na2O are formed by the complete reaction of 35.0 g of O2? 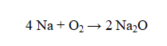
(Multiple Choice)
4.8/5  (38)
(38)
What is the percent yield when 0.750 mol of calcium reacts with sufficient oxygen to produce 34.4 g of CaO?
(Short Answer)
4.9/5  (34)
(34)
A reaction has a theoretical yield of 48.23 g. It produces 28.81 g of product. Calculate the percent yield.
(Short Answer)
4.9/5  (44)
(44)
In the reaction given below, if 12 moles of aluminum oxide are consumed, how many moles of oxygen gas are produced? 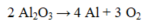
(Multiple Choice)
4.8/5  (41)
(41)
A combination reaction is considered the opposite of a(n) _____________ reaction.
(Short Answer)
4.8/5  (44)
(44)
In the reaction below, 8.0 g of H2 react with 7.0 g of O2. Which of the following statements is incorrect? 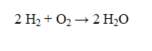
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following cannot be determined from a balanced chemical equation?
(Multiple Choice)
4.8/5  (44)
(44)
How many moles of oxygen will be produced if 27.6 grams of Al are produced? 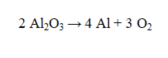
(Multiple Choice)
4.8/5  (32)
(32)
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 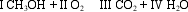
(Multiple Choice)
4.9/5  (42)
(42)
If 935 g of cesium reacts with excess chlorine to produce 1036 g of cesium chloride, what is the percent yield for the reaction? 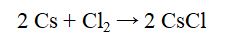
(Multiple Choice)
4.9/5  (38)
(38)
Which statement about the expression 6 C6H12O6 is not true?
(Multiple Choice)
4.7/5  (37)
(37)
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 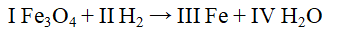
(Multiple Choice)
4.8/5  (29)
(29)
For the reaction given below, how many moles of aluminum will react if 12 moles of Br2 react and 8 moles of AlBr3 are produced? 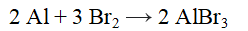
(Multiple Choice)
4.9/5  (33)
(33)
The Roman numerals in the reaction given represent the coefficients in the balanced chemical equation. What are the values of the coefficients? 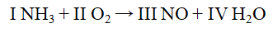
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)