Exam 17: Thermodynamics: Directionality of Chemical Reactions
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
If a reaction is product-favored at any temperature, then DH is ____ and DS is ____.
(Multiple Choice)
4.9/5  (37)
(37)
Consider the options given for the distribution of three units of energy to three particles. Which arrangement is least likely?
(Multiple Choice)
4.8/5  (39)
(39)
Since no chemical process is 100% efficient, some Gibbs free energy is always "wasted" and converted to ____ instead of doing useful work.
(Multiple Choice)
4.7/5  (45)
(45)
Use the data given to calculate the value of DG rxn at 25 C for the reaction given below. DG f for CO(g) is -137.16 kJ/mol. 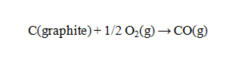
(Multiple Choice)
4.9/5  (46)
(46)
For a particular reaction, the value of DH = +98.8 kJ and DS = +141.5 J/K. This reaction is
(Multiple Choice)
4.8/5  (35)
(35)
DS for a reaction can be calculated from the following equation.
(Multiple Choice)
4.8/5  (36)
(36)
A reaction is exothermic and has a negative value of DS . The value of DG for this reaction is therefore:
(Multiple Choice)
5.0/5  (36)
(36)
At constant T and P, in which situation will the reaction be product-favored at high temperature but not at low temperature?
(Multiple Choice)
4.8/5  (37)
(37)
Use the data given to calculate the value of K for the reaction at 5 C. 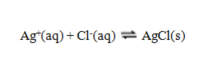

(Multiple Choice)
4.8/5  (35)
(35)
For a specific chemical reaction, the value of DG calculated from tabulated values of the standard Gibbs free energy of formation, DG f, of the reactants and products is valid at
(Multiple Choice)
4.8/5  (37)
(37)
Five coins are tossed. Which combination of heads (H) and tails (T) is least likely?
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the value of DS for the reaction shown: 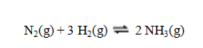 At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
(Multiple Choice)
4.8/5  (41)
(41)
The value of DG rxn at 45 C is -47.8 kJ. What is the value of the equilibrium constant for this reaction?
(Multiple Choice)
4.9/5  (44)
(44)
Two substances, A and B, have identical enthalpies of vaporization. They boil at 164 C and 236 C, respectively. If the entropy of vaporization of A is 87.2 J/K, what is the entropy of vaporization of B, in J/K?
(Multiple Choice)
4.8/5  (29)
(29)
How many of the following processes involve a decrease in disorder: humidifying dry air; distilling crude oil into gasoline, fuel oil, and jet fuel; filtering solid impurities out of a mixture; adding sand to water?
(Multiple Choice)
4.7/5  (41)
(41)
At a particular temperature, a reactant-favored process is one in which
(Multiple Choice)
4.9/5  (35)
(35)
A reaction cannot change between being product-favored and being reactant-favored when
(Multiple Choice)
4.9/5  (46)
(46)
Showing 21 - 40 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)