Exam 5: Introduction to Reactions in Aqueous Solutions
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
In an acid-base neutralization reaction,38.74 mL of 0.500 mol L-1 potassium hydroxide reacts with 50.00 mL of sulfuric acid solution.What is the concentration of the H2SO4 solution?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following pairs of aqueous solutions will give a precipitate when mixed?
(Multiple Choice)
4.8/5  (38)
(38)
According to the balanced equation shown below,4.00 moles of oxalic acid,H2C2O4,reacts with ________ moles of permanganate,MnO4-.
5 H2C2O4(aq)+ 2 MnO4-(aq)+ 6 H+(aq)→ 10 CO2(g)+ Mn2+(aq)+ 8 H2O(l)
(Multiple Choice)
4.8/5  (34)
(34)
Select a statement that best describes the oxidation process.
(Multiple Choice)
4.8/5  (32)
(32)
Indicate whether a precipitate forms by completing equation
Na+(aq)+ Cl-(aq)+ NO3-(aq)+ K+(aq)→ ?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following,in aqueous solution,is most likely to behave as a weak acid?
(Multiple Choice)
4.9/5  (38)
(38)
How many millilitres of 0.550 mol L-1 hydriodic acid are needed to react with 15.00 mL of 0.217 mol L-1 CsOH?
HI(aq)+ CsOH(aq)→ CsI(aq)+ H2O(l)
(Multiple Choice)
4.8/5  (31)
(31)
In which of the following pairs is the oxidation number for the underlined element INCORRECT?
(Multiple Choice)
5.0/5  (33)
(33)
The neutralization of 25.0 mL of 0.24 M HCl requires 5.0 mL of NaOH.What is the molarity of the NaOH solution?
(Multiple Choice)
4.8/5  (34)
(34)
What concentration of Fe2(SO4)3(aq)is required for the solution to have [Fe3+(aq)] = 0.32 M?
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following compounds is quite insoluble in water,but would liberate a gas when treated with aqueous acetic acid?
(Multiple Choice)
4.9/5  (34)
(34)
An insoluble compound will dissolve to an appreciable amount in water.
(True/False)
4.7/5  (37)
(37)
Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 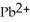 (aq)+
(aq)+ 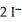 (aq)→
(aq)→ 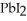 (s)
Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many millilitres of 3.550 mol L-1 HI(aq)must be added to a solution containing 0.700 mol of
(s)
Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many millilitres of 3.550 mol L-1 HI(aq)must be added to a solution containing 0.700 mol of 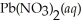 To completely precipitate the lead?
To completely precipitate the lead?
(Multiple Choice)
4.8/5  (26)
(26)
How many electrons are transferred when the perchlorate ion is converted to chloride?
(Multiple Choice)
4.8/5  (38)
(38)
Balance the following oxidation-reduction reaction in acid solution:
Fe(s)→ Fe2+ + H2(g)
The sum of the coefficients is ________.
(Multiple Choice)
4.9/5  (33)
(33)
Balance the following equation in basic solution:
Br2 + Mn2+ → MnO2 + Br-
The sum of the coefficients is ________.
(Multiple Choice)
4.9/5  (27)
(27)
Showing 81 - 100 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)