Exam 12: Intermolecular Forces: Liquids and Solids
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
Cesium has a radius of 272 pm and crystallizes in a body-centred cubic structure.What is the edge length of the unit cell?
(Multiple Choice)
5.0/5  (42)
(42)
Arrange the following compounds in order of increasing viscosity:
I.1-propanol,CH3CH2CH2OH;
II.1,2-propanediol,CH3CH(OH)CH2OH;
III.1,2,3-propanetriol, (glycerol),HOCH2CH(OH)CH2OH.
(Multiple Choice)
4.8/5  (46)
(46)
Lithium crystallizes in a body-centred cubic structure.What is the coordination number of each atom?
(Multiple Choice)
4.9/5  (37)
(37)
Which probably has the highest boiling point at 1.00 atm pressure?
(Multiple Choice)
4.9/5  (38)
(38)
Three types of holes of the cubic closest packed structure are:
(Multiple Choice)
4.8/5  (32)
(32)
When the vapor pressure of a liquid equals atmospheric pressure,the temperature of the liquid equals ________.
(Multiple Choice)
4.9/5  (37)
(37)
Given the following information,calculate ΔH° (in kcal mol-1)for:
Cl(g)+ e- → Cl-(g)

(Multiple Choice)
4.8/5  (40)
(40)
A 0.90 g sample of liquid water was introduced into an evacuated 2.00 L flask,which was then sealed and heated to 37 °C.What percentage,by mass,of the water remained as liquid? [Vapor pressure of water at 37 °C = 48.2 Torr.]
(Multiple Choice)
4.9/5  (36)
(36)
The phenomenon in which a steel needle can,with proper care,be made to float on the surface of some water illustrates a property of liquids known as:
(Multiple Choice)
4.9/5  (36)
(36)
Under which of the following conditions will vaporization best occur?
(Multiple Choice)
4.8/5  (30)
(30)
The heat of fusion for napthalene (C10H8)is 18.98 kJ/mol and for sodium is 2.60 kJ/mol.The amount of heat that would melt 37.0 grams of napthalene would melt how many grams of sodium?
(Multiple Choice)
4.9/5  (35)
(35)
According to the phase diagram given,which of the following statements is INCORRECT? 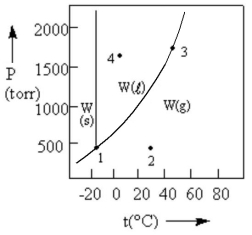
(Multiple Choice)
4.8/5  (33)
(33)
The property that causes water to have a concave meniscus but mercury to have a convex meniscus is ________.
(Multiple Choice)
4.8/5  (39)
(39)
Which of the substances below would produce the hardest crystals?
(Multiple Choice)
4.8/5  (39)
(39)
Given the following information,calculate ΔH° (in kcal mol-1)for: LiCl(s)→ Cl-(g)+ Li+(g)
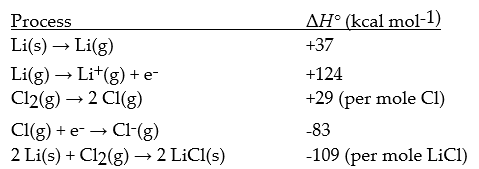
(Multiple Choice)
4.7/5  (38)
(38)
A liquid has a molar heat of vaporization of 22.7 kJ/mol.Its normal boiling point is 459 K.What is the vapor pressure,in mmHg,at 70 °C?
(Multiple Choice)
4.8/5  (30)
(30)
There are no types of crystalline solids that are held together by:
(Multiple Choice)
4.8/5  (37)
(37)
Showing 21 - 40 of 121
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)