Exam 17: Additional Aspects of Aqueous Equilibria
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
A 25.0-mL sample of 0.150 M hydrocyanic acid is titrated with a 0.150 M NaOH solution.What is the pH before any base is added? The 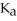 of hydrocyanic acid is 4.9 × 10-10.
of hydrocyanic acid is 4.9 × 10-10.
(Multiple Choice)
4.9/5  (28)
(28)
For which salt should the aqueous solubility be most sensitive to pH?
(Multiple Choice)
4.8/5  (38)
(38)
What is the percent ionization of nitrous acid in a solution that is 0.222 M in nitrous acid (HNO2)and 0.278 M in potassium nitrite (KNO2)? The acid dissociation constant of nitrous acid is 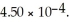
(Multiple Choice)
4.7/5  (41)
(41)
Which one of the following pairs cannot be mixed together to form a buffer solution?
(Multiple Choice)
4.8/5  (31)
(31)
In a solution,when the concentrations of a weak acid and its conjugate base are equal,________.
(Multiple Choice)
4.8/5  (39)
(39)
Decreasing the pH of blood will cause hemoglobin to release ________.
(Multiple Choice)
4.9/5  (36)
(36)
The Ksp for Cu(OH)2 is 4.8 × 10-20.Determine the molar solubility of Cu(OH)2 in a buffer solution with a pH of 10.1.
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the pH of a buffer solution that contains 0.010 moles of A- and 0.010 moles of HA in 100 ml of water.The Ka of HA is 1.77 × 10-5.
(Short Answer)
4.7/5  (30)
(30)
A solution is prepared by dissolving 0.23 mol of hypochlorous acid and 0.27 mol of sodium hypochlorite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 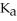 of hypochlorous acid is 1.36 × 10-3.
of hypochlorous acid is 1.36 × 10-3.
(Multiple Choice)
4.9/5  (35)
(35)
How many milliliters of 0.120 M NaOH are required to titrate 50.0 mL of 0.0998 M hypochlorous acid to the equivalence point? The 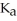 of hypochlorous acid is 3.0 × 10-8.
of hypochlorous acid is 3.0 × 10-8.
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the pH of a solution prepared by dissolving 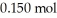 of acetic acid and
of acetic acid and 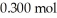 of sodium acetate in water sufficient to yield
of sodium acetate in water sufficient to yield 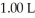 of solution.The Ka of acetic acid is
of solution.The Ka of acetic acid is 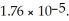
(Multiple Choice)
4.7/5  (37)
(37)
A 25.0 mL sample of an HCl solution is titrated with a 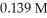 NaOH solution.The equivalence point is reached with
NaOH solution.The equivalence point is reached with 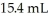 of base.The concentration of HCl is ________ M.
of base.The concentration of HCl is ________ M.
(Multiple Choice)
4.8/5  (39)
(39)
The pH of a solution prepared by mixing 50.0 mL of 0.125 M KOH and 50.0 mL of 0.125 M HCl is ________.
(Multiple Choice)
4.9/5  (30)
(30)
In which one of the following solutions is silver chloride the most soluble?
(Multiple Choice)
4.9/5  (42)
(42)
The pH of a solution prepared by mixing 45.0 mL of 0.183 M KOH and 35.0 mL of 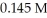 HCl is ________.
HCl is ________.
(Multiple Choice)
5.0/5  (39)
(39)
Calculate the maximum concentration (in M)of silver ions (Ag+)in a solution that contains 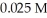 of CO32-.The Ksp of Ag2CO3 is
of CO32-.The Ksp of Ag2CO3 is 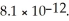
(Multiple Choice)
4.7/5  (42)
(42)
Showing 21 - 40 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)