Exam 17: Additional Aspects of Aqueous Equilibria
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The solubility of manganese (II)hydroxide (Mn(OH)2)is 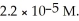 What is the Ksp of Mn(OH)2?
What is the Ksp of Mn(OH)2?
(Multiple Choice)
4.8/5  (34)
(34)
The solubility of a slightly soluble salt is increased by the presence of a second solute that provides a common ion to the system.
(True/False)
4.8/5  (39)
(39)
The addition of hydrofluoric acid and ________ to water produces a buffer solution.
(Multiple Choice)
4.8/5  (37)
(37)
The solubility of lead (II)chloride (PbCl2)is 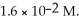 What is the Ksp of PbCl2?
What is the Ksp of PbCl2?
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the pH of a solution prepared by dissolving 0.270 mol of weak acid HA and 0.260 mol of its conjugate base in water sufficient to yield 1.00 L of solution.The 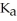 of HA is
of HA is 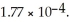
(Multiple Choice)
4.9/5  (31)
(31)
The Ka of benzoic acid is 6.30 × 10-5.The pH of a buffer prepared by combining 50.0 mL of 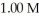 potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
(Multiple Choice)
4.8/5  (28)
(28)
Determine the Ksp for magnesium hydroxide (Mg(OH)2)where the solubility of Mg(OH)2 is 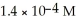 .
.
(Multiple Choice)
4.7/5  (40)
(40)
A buffer solution with a pH of 4.63 is prepared with 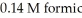 acid and
acid and 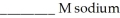 formate. The
formate. The 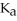 of formic acid is
of formic acid is 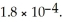
(Multiple Choice)
4.8/5  (36)
(36)
A 25.0 mL sample of 0.150 M acetic acid is titrated with a 0.150 M NaOH solution.What is the pH at the equivalence point? The 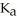 of acetic acid is 4.50 × 10-4.
of acetic acid is 4.50 × 10-4.
(Multiple Choice)
4.8/5  (30)
(30)
In which of the following aqueous solutions would you expect AgF to have the lowest solubility?
(Multiple Choice)
4.8/5  (29)
(29)
What change will be caused by addition of a small amount of HCl to a solution containing fluoride ions and hydrogen fluoride?
(Multiple Choice)
4.9/5  (38)
(38)
The addition of HF and ________ to water produces a buffer solution.
(Multiple Choice)
4.9/5  (33)
(33)
Calculate the pH of a solution that is 0.278 M in sodium formate ( 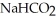 )and
)and 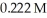 in formic acid
in formic acid 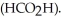 The
The 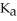 of formic acid is 1.77 ×
of formic acid is 1.77 × 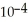 .
.
(Multiple Choice)
4.9/5  (32)
(32)
Suppose you have just added 500.0 ml of a solution containing 0.2000 moles of acetic acid per liter to 200.0 ml of 0.250 M NaOH.What is the final pH?
The Ka of acetic acid is 1.77 × 10-5.
(Short Answer)
5.0/5  (35)
(35)
A 25.0 mL sample of 0.150 M hydrazoic acid is titrated with a 0.150 M NaOH solution.What is the pH after 26.0 mL of base is added? The 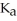 of hydrazoic acid is 1.9 × 10-5.
of hydrazoic acid is 1.9 × 10-5.
(Multiple Choice)
4.8/5  (30)
(30)
Of the substances below,________ will decrease the solubility of Pb(CN)2 in a saturated solution.
(Multiple Choice)
4.9/5  (35)
(35)
The concentration of fluoride ions in a saturated solution of barium fluoride is ________ M.The solubility product constant of BaF2 is 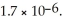
(Multiple Choice)
4.9/5  (31)
(31)
Calculate the pH of a solution prepared by dissolving 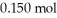 of benzoic acid and
of benzoic acid and 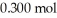 of sodium benzoate in water sufficient to yield
of sodium benzoate in water sufficient to yield 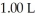 of solution.The Ka of benzoic acid is
of solution.The Ka of benzoic acid is 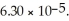
(Multiple Choice)
4.8/5  (32)
(32)
A 25.0 mL sample of 0.723 M HClO4 is titrated with a 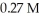 KOH solution.The H3O+ concentration after the addition of
KOH solution.The H3O+ concentration after the addition of 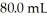 of KOH is ________ M.
of KOH is ________ M.
(Multiple Choice)
4.9/5  (39)
(39)
Showing 81 - 100 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)