Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Would you expect the following compound to absorb visible light? Why or why not? 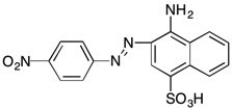
(Essay)
4.8/5  (34)
(34)
In the allyl radical, which π molecular orbital is singly occupied?
(Multiple Choice)
4.9/5  (37)
(37)
Draw the structure of the major product which results when the diene shown is treated with HBr at 40°C. 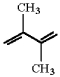
(Essay)
4.8/5  (38)
(38)
According to the 1,3-butadiene structure below, which positions would be best to place methoxy groups to yield the most reactive dimethoxy-1,3-butadiene isomer in the Diels-Alder reaction? 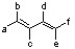
(Multiple Choice)
4.8/5  (35)
(35)
Draw structures for the two major products of the following reaction. 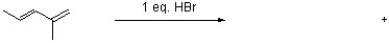
(Essay)
4.8/5  (33)
(33)
Draw the resonance structures of the intermediate and then predict the two major products in the following reaction. 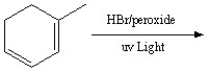
(Essay)
4.9/5  (39)
(39)
Indicate whether the following cyclization, conducted under thermal conditions, is symmetry allowed. Justify your answer by showing whether the overlapping orbitals in the transition state generate a symmetry-allowed or symmetry-forbidden scenario. Treat ethylene as the nucleophile. 
(Essay)
4.8/5  (37)
(37)
Show how the carbon p orbitals overlap to form the lowest energy π molecular orbital of 1,3-butadiene.
(Essay)
4.9/5  (33)
(33)
Provide the structure of the major organic product in the following reaction. 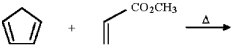
(Essay)
4.7/5  (34)
(34)
When the relative energies of the s-cis and s-trans conformers of 1,3-butadiene are compared, one finds that ________.
(Multiple Choice)
4.9/5  (43)
(43)
The Diels-Alder reaction is a concerted reaction; this means ________.
(Multiple Choice)
4.8/5  (41)
(41)
How many nodes, other than the node coincident with the molecular plane, are found in the highest energy π MO of 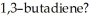
(Multiple Choice)
4.9/5  (43)
(43)
Which substrate would react most rapidly in an SN2 reaction?
(Multiple Choice)
4.9/5  (32)
(32)
How many electrons are present in the nonbonding π molecular orbital of the allyl anion?
(Multiple Choice)
4.7/5  (46)
(46)
Provide the necessary synthetic sequence for preparing 1,3-cyclopentadiene from cyclopentane.
(Essay)
4.7/5  (28)
(28)
What name is given to the type of "control" which arises in a reaction which does not achieve equilibrium and in which the product distribution is determined by the relative activation energies of the pathways which produced them?
(Short Answer)
4.8/5  (39)
(39)
Why does the diene shown below fail to undergo a Diels-Alder reaction with even the most reactive dienophiles? 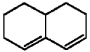
(Essay)
4.8/5  (38)
(38)
Including all possible stereoisomeric forms, how many distinct allylic bromides could be produced when 2-methylpent-1-ene is treated with NBS under irradiation by a sunlamp?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 60 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)