Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
In the diene shown below, there are two possible double bonds that could react when 1 eq. of HBr is added. However, the double bond labeled "A" reacts preferentially to give the major product. Using resonance structures, justify this observation. 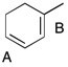
(Essay)
4.8/5  (42)
(42)
What diene and dienophile are used in the Diels-Alder route to the compound shown? 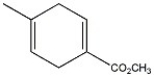
(Essay)
4.8/5  (37)
(37)
Explain why SN2 reactions on allyl bromide proceed faster than corresponding reactions on ethyl bromide.
(Essay)
4.9/5  (43)
(43)
Give a representation of the antibonding π molecular orbital of the allyl cation.
(Essay)
4.9/5  (40)
(40)
What is the hybridization of the central carbon of allene (1,2-propadiene)?
(Multiple Choice)
4.8/5  (36)
(36)
Give a representation of the bonding π molecular orbital of the allyl anion.
(Essay)
4.8/5  (38)
(38)
Among a series of isomeric trienes, the more negative the ΔH° of the hydrogenation reaction of a given triene, the ________ stable it is relative to the others in the isomeric series.
(Short Answer)
4.9/5  (45)
(45)
Name the two major products which are formed when 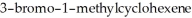 undergoes solvolysis in hot methanol.
undergoes solvolysis in hot methanol.
(Essay)
4.9/5  (34)
(34)
What diene and dienophile are used in the Diels-Alder route to the compound shown? 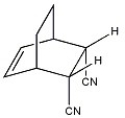
(Essay)
4.8/5  (42)
(42)
Rank the following dienes in order of increasing stability: trans-1,3-pentadiene, cis-1,3-pentadiene, 1,4-pentadiene, and 1,2-pentadiene.
(Essay)
4.9/5  (41)
(41)
The following Diels-Alder reaction was featured in Tet. Lett. 2011, 960. Predict the structure of the starting materials. 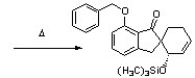
(Essay)
4.9/5  (34)
(34)
Given that 1,3-butadiene has a uv absorption of 217nm, predict the approximate absorption for the conjugated system in vitamin D3. 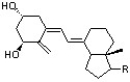
(Multiple Choice)
4.9/5  (37)
(37)
Provide the structure of the major product which results from 1,2-addition of HBr to the diene shown below. 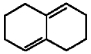
(Essay)
4.9/5  (30)
(30)
Showing 101 - 120 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)