Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
In 1,3,5-hexatriene, there are ________ nodes in the HOMO and ________ nodes in the LUMO for this molecule.
(Multiple Choice)
4.9/5  (47)
(47)
Anthocyanidins are the organic compounds largely responsible for the color of wine and grape juice. They can also be used as indicators in pH titrations. Given the reaction below, which form of the compound is colored and which is colorless. 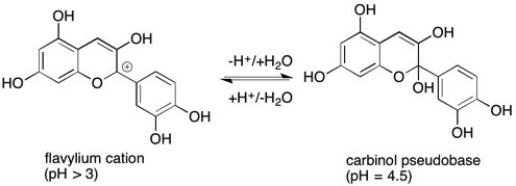
(Essay)
5.0/5  (39)
(39)
Draw the structure of the major product which results when the diene shown is treated with HBr at -80°C. 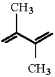
(Essay)
4.8/5  (46)
(46)
Absorption of UV-visible energy by a molecule results in ________.
(Multiple Choice)
4.8/5  (34)
(34)
Provide the structure of the major organic product in the following reaction. 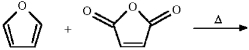
(Essay)
4.9/5  (34)
(34)
Show how the carbon p orbitals overlap to form the LUMO of the allyl anion.
(Essay)
4.8/5  (33)
(33)
When (S)-3-bromopent-1-ene is heated in water, which of the following compounds is not produced?
(Multiple Choice)
4.9/5  (30)
(30)
Is the thermal [4+2] cycloaddition between allyl anion and ethylene an allowed one? To answer, draw the HOMO of allyl anion and the LUMO of ethylene, and comment on the symmetry match of the two.
(Essay)
4.8/5  (38)
(38)
When 1 mole of anhydrous HCl is reacted with excess 1,3-pentadiene, both the 1,2 and the 1,4-addition products are formed. Which of the following structures shown below is the least likely to be one of these products? (Note: When a chiral carbon is formed in this reaction a racemic mixture results, only one of the two possible enantiomers is shown.)
(Multiple Choice)
4.8/5  (50)
(50)
In the addition of HBr to conjugated dienes, is the product which results from 1,2-addition or that which results from 1,4-addition typically the product of kinetic control?
(Essay)
4.8/5  (36)
(36)
Rank the following compounds in order of increasing heats of hydrogenation. (The smallest ΔH is first.) 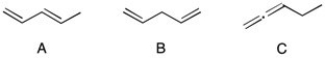
(Multiple Choice)
4.7/5  (38)
(38)
Provide the structure of the major product which results from 1,4-addition of Br2 to the diene shown below. 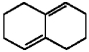
(Essay)
4.9/5  (40)
(40)
Which of the following represents the highest occupied molecular orbital for the conjugated pi system in Vitamin D3? 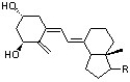
(Multiple Choice)
4.9/5  (37)
(37)
Showing 61 - 80 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)