Exam 1: Structure and Bonding
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
The element with the electronic configuration 1s22s22p63s1 is ________.
(Short Answer)
4.9/5  (32)
(32)
One resonance structure of a cation is shown. Provide the other reasonable resonance structures. 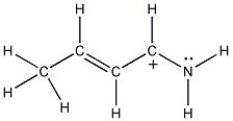
(Essay)
4.7/5  (42)
(42)
Which of the following condensed formulas represents the same compound as the line-angle structure shown? 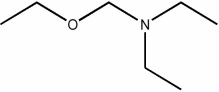
(Multiple Choice)
4.8/5  (39)
(39)
Provide the products of the following acid-base reaction.
(CH3)3NH+ + HO-→
(Essay)
4.9/5  (29)
(29)
Calculate the molecular formula for the organic compound whose quantitative elemental analysis showed 48.6% caron and 8.1% hydrogen by weight.
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following are acceptable Lewis structures, including formal charges, for nitric acid, HNO3? 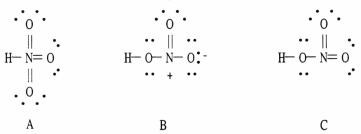
(Multiple Choice)
4.8/5  (35)
(35)
Draw the Lewis structure for 2-propanol, CH3CH(OH)CH3, including all non-bonding lone pairs.
(Essay)
4.8/5  (37)
(37)
Provide the line-angle formula (skeletal structure) for (CH3CH2)2C=O.
(Essay)
4.9/5  (41)
(41)
The pH of a 150 mL aqueous solution of 2.13 x 10-3 M HCl is ________.
(Multiple Choice)
4.9/5  (45)
(45)
When filling two or more orbitals of the same energy with electrons, the electrons will go into different orbitals rather than pair up in the same orbital.
(True/False)
4.9/5  (39)
(39)
Draw the Lewis structure for boric acid, B(OH)3, including all non-bonding lone pairs.
(Essay)
4.8/5  (31)
(31)
Stabilization of a charged species usually results when this species can be more accurately depicted as a hybrid of several resonance forms. Why is this the case?
(Essay)
4.8/5  (41)
(41)
The compound phenol is shown below. Provide the structure of the conjugate base of phenol. 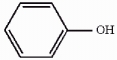
(Essay)
4.7/5  (38)
(38)
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 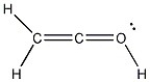
(Essay)
4.8/5  (39)
(39)
Indicate the line-angle structure that corresponds to the condensed structure, HOCH2C(O)CH(CH3)2.
(Multiple Choice)
4.8/5  (35)
(35)
Covalent bonds may be polar or nonpolar. What property of the atoms forming a given bond determines this?
(Short Answer)
4.8/5  (41)
(41)
Which of the following structures (a-d) is another resonance structure of the following organic molecule? 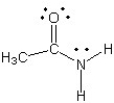
(Multiple Choice)
4.7/5  (38)
(38)
Showing 21 - 40 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)