Exam 1: Structure and Bonding
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Atoms with the same number of protons but different numbers of neutrons are called ________.
(Short Answer)
4.8/5  (34)
(34)
The Ka of formic acid is 1.7 x 10-4. The pKa of formic acid is ________.
(Multiple Choice)
4.9/5  (34)
(34)
Which sequence correctly ranks the following protons in order of increasing acidity? 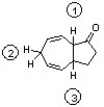
(Multiple Choice)
4.9/5  (38)
(38)
Peramivir, shown below, has shown to be effective against the influenza B virus (J. Med. Chem. 2010, 6421). Which sequence ranks the following nitrogen atoms in order of increasing basicity? 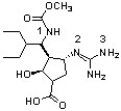
(Multiple Choice)
4.9/5  (37)
(37)
Draw the line-angle formula for three compounds with molecular formula C3H8O.
(Essay)
4.8/5  (32)
(32)
Which of the following bonding patterns of carbon is not allowed in the formation of an organic compound?
(Multiple Choice)
5.0/5  (41)
(41)
Draw 2 possible Lewis structures for the compound with molecular formula C3H6.
(Essay)
4.9/5  (32)
(32)
Draw a complete Lewis structure, including lone pairs, for (CH3)2CHCO2H.
(Essay)
4.9/5  (47)
(47)
Compute the empirical and molecular formulas for the compound of molecular weight 86 g/mol which is shown to contain 55.8% C and 7.0% H by elemental analysis.
(Essay)
4.9/5  (34)
(34)
Orbitals which are equal in energy are referred to as ________.
(Multiple Choice)
4.7/5  (40)
(40)
The formal charge on the nitrogen on the structure shown below is: 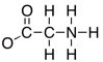
(Short Answer)
4.8/5  (42)
(42)
The Lewis structure of trimethylamine is shown below. Draw the condensed structural formula which corresponds to this Lewis structure. 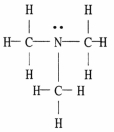
(Short Answer)
4.8/5  (44)
(44)
Which of the following terms comes closest to describing an electrophile?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following acids has the most stable conjugate base?
(Multiple Choice)
4.8/5  (29)
(29)
Rank the following compounds in order of increasing basicity: CH3O-, H2N-, H2O, and NH3.
(Essay)
4.8/5  (35)
(35)
When a molecule can best be represented as a series of resonance forms, each of these forms always contributes to the same degree in the hybrid.
(True/False)
4.8/5  (39)
(39)
Which is more acidic, methanesulfonic acid (CH3SO3H) or propanoic acid (CH3CH2CO2H)? Explain.
(Essay)
4.7/5  (29)
(29)
When methanol (CH3OH) acts as a base, its conjugate acid is ________.
(Multiple Choice)
4.9/5  (31)
(31)
A node is a region of high electron density between the two atoms in a covalent bond.
(True/False)
4.8/5  (42)
(42)
Showing 61 - 80 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)