Exam 1: Structure and Bonding
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Draw condensed structures for the four compounds with formula C3H9N.
(Essay)
4.7/5  (35)
(35)
Rank the following compounds in order of increasing acidity: CH3OH, HCl, NH3, and CH4.
(Essay)
4.9/5  (33)
(33)
The formal charge on the oxygens in the compound below are ________. 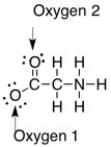
(Multiple Choice)
4.7/5  (38)
(38)
The ________ tells us that each orbital can hold a maximum of 2 electrons.
(Multiple Choice)
4.9/5  (24)
(24)
Add the appropriate formal charge to each atom in the molecule below. It is not necessary to indicate formal charges when zero. (All non-bonding electrons are included.) 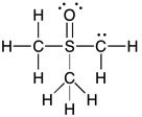
(Essay)
4.8/5  (46)
(46)
In the compound sodium methoxide (NaOCH3), there is ________ bonding.
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following condensed formulas correctly represents the line-angle structure shown below? 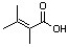
(Multiple Choice)
4.9/5  (44)
(44)
Assign the correct formal charge to each nitrogen atom in the following Lewis structure. (All non-bonding electrons are included.) 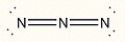
(Essay)
4.9/5  (35)
(35)
Use the curved arrow formalism to indicate the movement of electron pairs in the following reaction. 
(Essay)
4.7/5  (32)
(32)
While you were up late one night studying organic chemistry, you happened to see the last 5 minutes of an infomercial on TV. The spokesperson claimed that their brand of automobile tires were superior to all other brands on the market because they were made by using only natural rubber, isolated from the resin of rubber trees. How could a chemist test her claims that no petroleum products went into the manufacture of her brand of tires?
(Essay)
4.7/5  (45)
(45)
Provide the line-angle formula (skeletal structure) for (CH3)2CHCH2CHO.
(Essay)
4.8/5  (38)
(38)
The electronegativity of elements on the periodic table increases going ________ a column and to the ________ in each row.
(Multiple Choice)
4.8/5  (28)
(28)
In the following acid/base reaction, label the acid, base, conjugate acid and conjugate base.
HO- + (CH3)3NH+ → H2O + (CH3)3N
(Essay)
4.9/5  (35)
(35)
Which element in the second row of the periodic table has six valence electrons and a valence of two?
(Short Answer)
4.8/5  (44)
(44)
Write a completed equation for the acid-base pair shown below.
HCO2H + -NH2 →
(Essay)
4.8/5  (38)
(38)
The hydroxide ion (HO-) cannot function well as which of the following?
(Multiple Choice)
4.7/5  (30)
(30)
What is the molecular formula for the following line-angle structure? 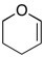
(Short Answer)
4.7/5  (38)
(38)
Showing 101 - 120 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)