Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Consider the reaction Cu2+(aq)+ 4 NH3(aq) 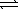 Cu(NH3)42+(aq)
Kf = 2.1 × 1013
If the Ksp for Cu(OH)2 is 2.2 × 10-20,what is the value of the equilibrium constant,K,for the reaction below?
Cu(NH3)42+(aq)+ 2 OH-(aq)
Cu(NH3)42+(aq)
Kf = 2.1 × 1013
If the Ksp for Cu(OH)2 is 2.2 × 10-20,what is the value of the equilibrium constant,K,for the reaction below?
Cu(NH3)42+(aq)+ 2 OH-(aq) 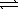 Cu(OH)2(s)+ 4 NH3(aq)
Cu(OH)2(s)+ 4 NH3(aq)
(Multiple Choice)
4.9/5  (43)
(43)
A solution containing 10.mmol of CO32−and 5.0 mmol of HCO3−is titrated with 1.1 M HCl.What volume of HCl must be added to reach the first equivalence point? (1 mmol = 0.001 mol)
(Multiple Choice)
5.0/5  (40)
(40)
An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below. 2 HCl(aq)+ Na2CO3(aq) 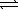 CO2(g)+ H2O(
CO2(g)+ H2O( 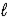 )+ 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
)+ 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
(Multiple Choice)
4.8/5  (39)
(39)
A 25.00-mL sample of propionic acid,HC3H5O2,of unknown concentration was titrated with 0.145 M KOH.The equivalence point was reached when 43.02 mL of base had been added.What was the original concentration of the propionic acid?
(Multiple Choice)
4.8/5  (29)
(29)
To make a buffer with a pH of 8.00,you should use a weak acid with a Ka close to _______.
(Short Answer)
4.9/5  (45)
(45)
What is the pH of a saturated solution of Fe(OH)2? (Ksp = 8.0 × 10-16 and Kw = 1.01 × 10-14)
(Multiple Choice)
4.9/5  (45)
(45)
The concentration of Pb2+ in an aqueous solution is 5.5 × 10-3 M.What concentration of SO42- is required to begin precipitating PbSO4? The Ksp of PbSO4 is 2.5 × 10-8.
(Multiple Choice)
4.8/5  (32)
(32)
What is the pH of the buffer that results when 12.0 g of NaH2PO4 and 8.00 g of Na2HPO4 are diluted with water to a volume of 0.50 L? (Ka of H2PO4- = 6.2 × 10-8,the molar masses of NaH2PO4 and Na2HPO4 are 120.0 g/mol and 142.0 mol,respectively)
(Multiple Choice)
4.9/5  (31)
(31)
What is the pH of a solution made by combining 134 mL of 0.26 M NaC2H3O2 with 211 mL of 0.31 M HC2H3O2? The Ka of acetic acid is 1.75 × 10-5.
(Multiple Choice)
4.8/5  (30)
(30)
A solution containing 10.mmol of CO32− and 5.0 mmol of HCO3−is titrated with 1.1 M HCl.What total volume of HCl must be added to reach the second equivalence point? (1 mmol = 0.001 mol)
(Multiple Choice)
4.9/5  (37)
(37)
A buffer is composed of 0.400 mol H2PO4- and 0.400 mol HPO42- diluted with water to a volume of 1.00 L.The pH of the buffer is 7.210.How many moles of HCl must be added to decrease the pH to 6.210?
(Multiple Choice)
4.8/5  (41)
(41)
For a monoprotic acid titration,the _____ in a titration is the point where number of moles of a strong base added equals the number of moles of an acid initially present.
(Short Answer)
4.8/5  (33)
(33)
A 25.0 mL sample of 0.10 M sodium benzoate is titrated with 0.10 M HCl(aq).What is the pH after the addition of 32.0 mL of HCl(aq)? (Kb of C6H5CO2- = 1.6 × 10-10)
(Multiple Choice)
4.8/5  (35)
(35)
Titration of 0.1615 g of an unknown monoprotic acid dissolved in 25.00 mL of water requires 21.84 mL of 0.1231 M NaOH to reach the endpoint.What is the molar mass of the acid?
(Multiple Choice)
4.9/5  (41)
(41)
In which of the following solutions would silver(I)phosphate,Ag3PO4,be least soluble?
(Multiple Choice)
4.9/5  (36)
(36)
The pH of a solution at the equivalence point in a monoprotic strong acid-−strong base reaction is always 7.0 at 25 °C.
(True/False)
4.7/5  (37)
(37)
When mixed in appropriate amounts,each of the following mixtures can produce an effective buffer solution except _____.
(Multiple Choice)
4.9/5  (33)
(33)
If the ratio of acid to base in a buffer increases by a factor of 10,the pH of the buffer
(Multiple Choice)
4.9/5  (38)
(38)
What is the pH of a buffer that results when 0.50 mole of H3PO4 is mixed with 0.25 mole of NaOH and diluted with water to 1.00 L? (The acid dissociation constants of phosphoric acid are Ka1 = 7.5 × 10-3,Ka2 = 6.2 × 10-8,and Ka3 = 3.6 × 10-13)
(Multiple Choice)
4.7/5  (42)
(42)
Given the following reactions, AgBr(s) 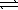 Ag+(aq)+ Br-(aq)
Ksp = 5.4 × 10-13
Ag+(aq)+ 2 CN-(aq)
Ag+(aq)+ Br-(aq)
Ksp = 5.4 × 10-13
Ag+(aq)+ 2 CN-(aq) 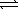 Ag(CN)2-(aq)
Kf = 1.2 × 1021
Determine the equilibrium constant for the reaction below.
AgBr(s)+ 2 CN-(aq)
Ag(CN)2-(aq)
Kf = 1.2 × 1021
Determine the equilibrium constant for the reaction below.
AgBr(s)+ 2 CN-(aq) 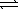 Ag(CN)2-(aq)+ Br-(aq)
Ag(CN)2-(aq)+ Br-(aq)
(Multiple Choice)
4.9/5  (44)
(44)
Showing 41 - 60 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)