Exam 2: Biochemistry Basics
Exam 1: Introduction to Microbiology46 Questions
Exam 2: Biochemistry Basics60 Questions
Exam 3: Introduction to Prokaryotic Cells47 Questions
Exam 4: Introduction to Eukaryotic Cells51 Questions
Exam 5: Genetics55 Questions
Exam 6: Viruses and Prions49 Questions
Exam 7: Fundamentals of Microbial Growth46 Questions
Exam 8: Microbial Metabolism60 Questions
Exam 9: Principles of Infectious Disease and Epidemiology47 Questions
Exam 10: Host-Microbe Interactions and Pathogenesis46 Questions
Exam 11: Innate Immunity60 Questions
Exam 12: Adaptive Immunity60 Questions
Exam 13: Immune System Disorders47 Questions
Exam 14: Vaccines and Biotechnology-Based Diagnostics and Therapeutics47 Questions
Exam 15: Antimicrobial Drugs46 Questions
Exam 16: Respiratory System Infections46 Questions
Exam 17: Skin and Eye Infections47 Questions
Exam 18: Nervous System Infections46 Questions
Exam 19: Digestive System Infections53 Questions
Exam 20: Urinary and Reproductive System Infections46 Questions
Exam 21: Cardiovascular and Lymphatic Infections46 Questions
Select questions type
A reversible reaction is one in which the forward and reversible reactions are both possible such as: AB -A + B and A + B -AB.
(True/False)
4.9/5  (31)
(31)
In the figure shown, which atom(s) will have a partial negative charge? 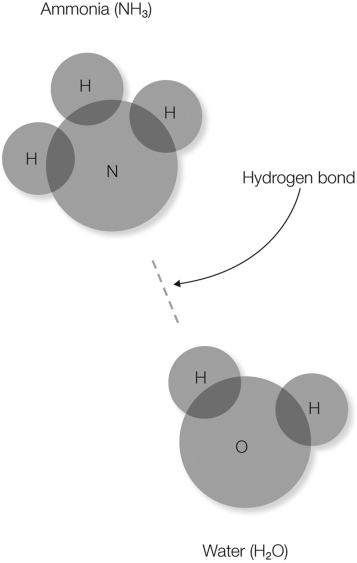
(Multiple Choice)
4.9/5  (30)
(30)
Which functional group is incorrectly matched with its structure?
(Multiple Choice)
4.8/5  (33)
(33)
Which type of lipid is incorrectly matched to its description?
(Multiple Choice)
4.9/5  (24)
(24)
Plasma membranes, the key boundary layer of cells, are composed of amphipathic molecules called phospholipids. Why would neither purely hydrophilic nor purely hydrophobic molecules be a suitable molecule for plasma membranes?
(Multiple Choice)
4.8/5  (36)
(36)
During vigorous exercise, both carbon dioxide and lactic acid enter the blood in increased amounts. Both compounds have the effect of lowering the blood pH. In order to maintain blood pH within the normal range of 7.35- 7.45, we would expect the carbonic acid (H2CO3) portion of the blood buffer system to pick up the extra H+ ions.
(True/False)
4.7/5  (46)
(46)
In a polar covalent bond involving hydrogen and oxygen, the hydrogen takes on a partial negative charge while the oxygen takes on a partial positive charge.
(True/False)
4.7/5  (39)
(39)
Deoxyribonucleotides and ribonucleotides differ in all of the following except
(Multiple Choice)
4.9/5  (31)
(31)
Vitamin deficiencies (not getting enough of a certain vitamin) pose obvious health problems, but an excess of certain vitamins in the system can also be harmful. Explain why you would be far less likely to experience an excess of water- soluble vitamins compared to fat- soluble vitamins in the context of the characteristics of polar and nonpolar substances.
(Essay)
4.9/5  (26)
(26)
Consider the reaction AB - A + B. What is the product of this reaction?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 41 - 60 of 60
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)