Exam 3: Energy and Enzymes
Exam 1: Light and Life109 Questions
Exam 2: The Cell: an Overview155 Questions
Exam 3: Energy and Enzymes66 Questions
Exam 4: Cell Membranes and Signaling81 Questions
Exam 5: Cellular Respiration61 Questions
Exam 6: Photosynthesis95 Questions
Exam 7: Cell Cycles93 Questions
Exam 8: Genetic Recombination88 Questions
Exam 9: The Chromosomal Basis of Mendelian Inheritance86 Questions
Exam 10: Genetic Linkage, Sex-Linkage, and Other Non-Mendelian Inheritance Mechanisms73 Questions
Exam 11: DNA Structure, Replication, and Repair57 Questions
Exam 12: Gene Structure Expression, and Mutation106 Questions
Exam 13: Regulation of Gene Expression91 Questions
Exam 14: Dna Technologies91 Questions
Exam 15: Genomes53 Questions
Exam 16: Evolution: the Development of the Theory66 Questions
Exam 17: Microevolution: Changes Within Populations83 Questions
Exam 18: Speciation and Macroevolution64 Questions
Exam 19: Systematics and Phylogenetics: Revealing the Tree of Life68 Questions
Exam 20: Humans and Evolution54 Questions
Exam 21: Defining Life and Its Origins55 Questions
Exam 22: Viruses, Viroids, and Prions: Infectious Biological Particles38 Questions
Exam 23: Bacteria and Archaea78 Questions
Exam 24: Protists98 Questions
Exam 25: Fungi81 Questions
Exam 26: Plants80 Questions
Exam 27: Animals171 Questions
Exam 28: Conservation of Biodiversity41 Questions
Exam 29: Population Ecology65 Questions
Exam 30: Species Interactions and Community Ecology70 Questions
Exam 31: Ecosystems68 Questions
Exam 32: Animal Behaviour120 Questions
Exam 33: Organization of the Plant Body69 Questions
Exam 34: Transport in Plants80 Questions
Exam 35: Reproduction and Development in Flowering Plants70 Questions
Exam 36: Plant Nutrition97 Questions
Exam 37: Plant Signals and Responses to the Environment93 Questions
Exam 38: Introduction to Animal Organization and Physiology65 Questions
Exam 39: Animal Nutrition98 Questions
Exam 40: Gas Exchange: the Respiratory System56 Questions
Exam 41: Internal Transport: the Circulatory System72 Questions
Exam 42: Regulation of the Internal Environment: Water, Solutes, and Temperature75 Questions
Exam 43: Control of Animal Processes: Endocrine Control80 Questions
Exam 44: Animal Reproduction168 Questions
Exam 45: Control of Animal Processes: Neural Control253 Questions
Exam 46: Muscles, Skeletons, and Body Movements71 Questions
Select questions type
Match each definition with the corresponding term.
-active site
A)primary coupling agent in cellular reactions
B)addition of a phosphate group to a target molecule
C)product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
D)linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
E)series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
F)substance that facilitates a chemical reaction without itself being consumed by the reaction
G)energy needed to start a reaction, be it endergonic or exergonic
H)portion of the enzyme that binds to a reactant or reactants
I)state in which the rate of the forward reaction equals the rate of the reverse reaction
J)intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
K)reactant molecule that binds to an enzyme
Free
(Short Answer)
4.9/5  (32)
(32)
Correct Answer:
h
Match each definition with the corresponding term.
-metabolic pathway
A)primary coupling agent in cellular reactions
B)addition of a phosphate group to a target molecule
C)product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
D)linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
E)series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
F)substance that facilitates a chemical reaction without itself being consumed by the reaction
G)energy needed to start a reaction, be it endergonic or exergonic
H)portion of the enzyme that binds to a reactant or reactants
I)state in which the rate of the forward reaction equals the rate of the reverse reaction
J)intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
K)reactant molecule that binds to an enzyme
Free
(Short Answer)
4.9/5  (34)
(34)
Correct Answer:
e
Match each definition with the corresponding term.
-activation energy
A)primary coupling agent in cellular reactions
B)addition of a phosphate group to a target molecule
C)product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
D)linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
E)series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
F)substance that facilitates a chemical reaction without itself being consumed by the reaction
G)energy needed to start a reaction, be it endergonic or exergonic
H)portion of the enzyme that binds to a reactant or reactants
I)state in which the rate of the forward reaction equals the rate of the reverse reaction
J)intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
K)reactant molecule that binds to an enzyme
Free
(Short Answer)
4.9/5  (39)
(39)
Correct Answer:
g
Which of the following best illustrates the first law of thermodynamics by Niagara Falls?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following occurs when a reaction reaches equilibrium?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following best describes a dead cell as a system?
(Multiple Choice)
4.9/5  (36)
(36)
Figure 3-5 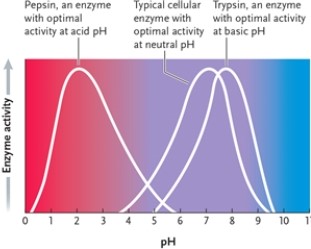 -According to the graph in Figure 3-5, what is the optimal pH for enzyme 1?
-According to the graph in Figure 3-5, what is the optimal pH for enzyme 1?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following can be said to occur during every energy transformation?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following best explains why machines can never be 100% efficient?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following best describes why bricks from a truck fall all over during a traffic accident?
(Multiple Choice)
4.8/5  (34)
(34)
AMP is the primary energy and phosphate source in coupled reactions.
(True/False)
4.8/5  (36)
(36)
Figure 3-1 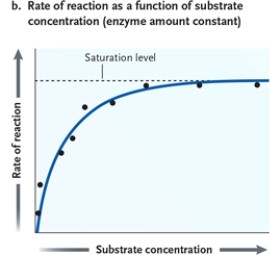 -Refer to Figure 3-1. Suppose you conduct an experiment in the laboratory in which you add increasing amounts of substrate to a solution containing an enzyme and a pH buffer. You incubate the container at the optimal temperature for the enzyme. Each time you add more substrate, you measure the rate of the reaction. Also suppose that you graph the results such that the x-axis shows the substrate concentration and the y-axis shows the resulting reaction rate. What will you find over time?
-Refer to Figure 3-1. Suppose you conduct an experiment in the laboratory in which you add increasing amounts of substrate to a solution containing an enzyme and a pH buffer. You incubate the container at the optimal temperature for the enzyme. Each time you add more substrate, you measure the rate of the reaction. Also suppose that you graph the results such that the x-axis shows the substrate concentration and the y-axis shows the resulting reaction rate. What will you find over time?
(Multiple Choice)
4.7/5  (28)
(28)
Match each definition with the corresponding term.
-ATP
A)primary coupling agent in cellular reactions
B)addition of a phosphate group to a target molecule
C)product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
D)linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
E)series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
F)substance that facilitates a chemical reaction without itself being consumed by the reaction
G)energy needed to start a reaction, be it endergonic or exergonic
H)portion of the enzyme that binds to a reactant or reactants
I)state in which the rate of the forward reaction equals the rate of the reverse reaction
J)intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
K)reactant molecule that binds to an enzyme
(Short Answer)
4.8/5  (31)
(31)
Which of the following is a correct pair based on their shared characteristics?
(Multiple Choice)
4.8/5  (44)
(44)
Match each definition with the corresponding term.
-transition state
A)primary coupling agent in cellular reactions
B)addition of a phosphate group to a target molecule
C)product of the reaction interacts with an enzyme in a noncompetitive way to inhibit or enhance enzyme activity
D)linking of an exergonic reaction with an endergonic reaction that allows a cell to drive a nonspontaneous reaction to completion
E)series of chemical reactions where the products of one reaction are the reactants for a subsequent reaction
F)substance that facilitates a chemical reaction without itself being consumed by the reaction
G)energy needed to start a reaction, be it endergonic or exergonic
H)portion of the enzyme that binds to a reactant or reactants
I)state in which the rate of the forward reaction equals the rate of the reverse reaction
J)intermediate arrangement of unstable bonds between atoms that can proceed toward either the reactants or the products of a reaction
K)reactant molecule that binds to an enzyme
(Short Answer)
4.9/5  (38)
(38)
Which of the following best describes why thinking of entropy as disorder is problematic?
(Multiple Choice)
4.8/5  (31)
(31)
Figure 3-5  -Suppose all three enzymes represented in Figure 3-5 catalyze the same reaction, but conditions require you to use a pH of 7. Which is the best enzyme to use?
-Suppose all three enzymes represented in Figure 3-5 catalyze the same reaction, but conditions require you to use a pH of 7. Which is the best enzyme to use?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)