Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Which one of the following lists gives the correct symbols for the elements phosphorus,potassium,silver,chlorine,and sulfur?
(Multiple Choice)
4.9/5  (38)
(38)
Lithium has two naturally occurring isotopes,6Li and 7Li .The average atomic mass of lithium is 6.941.Which of the following statements concerning the relative abundance of each isotope is correct?
(Multiple Choice)
4.8/5  (43)
(43)
The formulas of the carbonate ion,the ammonium ion,and the chlorate ion are represented,respectively,as
(Multiple Choice)
4.8/5  (43)
(43)
If the Thomson model of the atom had been correct,Rutherford would have observed
(Multiple Choice)
4.8/5  (30)
(30)
Energy from the following reaction provided the lift for the moon lander:
__ (CH3)2N2H2 + __ N2O4 → __ N2 + __ H2O + __ CO2
When the equation is balanced,the smallest whole-number coefficient of nitrogen is
(Multiple Choice)
4.7/5  (43)
(43)
The formulas of the hydroxide ion,the nitrate ion,and the phosphate ion are represented,respectively,as
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following is the atomic symbol for the element cobalt?
(Multiple Choice)
4.7/5  (37)
(37)
The mass spectrum of an element with two naturally occurring isotopes is shown below.What is the best estimate of the element's atomic mass? 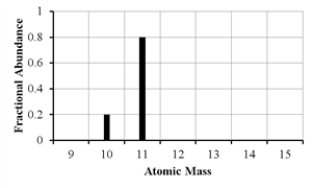
(Multiple Choice)
4.9/5  (36)
(36)
The chemical name for the binary,non-ionic molecule with the formula HI is
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following nuclides contains more protons than neutrons?
(Multiple Choice)
4.8/5  (42)
(42)
The complete combustion of propane,C3H8,yields carbon dioxide and water:
- C3 H8 + - O2→ - CO2 + - H2O
The smallest whole-number coefficient of oxygen in the balanced equation is
(Multiple Choice)
4.8/5  (30)
(30)
A 2.0-g sample of washing soda,Na2CO3 • 10H2O,has 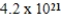 carbon atoms.How many oxygen atoms are present in 2.0g of washing soda?
carbon atoms.How many oxygen atoms are present in 2.0g of washing soda?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following elements has the largest atomic mass?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following pairs of compounds can be used to illustrate the law of multiple proportions?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 41 - 60 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)