Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
A certain element is listed as having an atomic mass of 63.5 amu.It is probably true that this element contains
(Multiple Choice)
4.8/5  (31)
(31)
The complete combustion of octane,C8H18,yields carbon dioxide and water:
- C8 H18 + - O2→ - CO2 + - H2O
The smallest whole-number coefficient of oxygen in the balanced equation is
(Multiple Choice)
4.9/5  (35)
(35)
The average atomic mass of Eu is 151.96 amu.There are only two naturally occurring isotopes of europium,151Eu with a mass of 151.0 amu and 153Eu with a mass of 153.0 amu.The natural abundance of the 131Eu isotope must be approximately
(Multiple Choice)
5.0/5  (43)
(43)
What is the ratio of oxygen atoms to hydrogen atoms in the mineral carnotite,K2(UO2)3(VO4)2 • 3H2O?
(Multiple Choice)
4.8/5  (35)
(35)
What is the name of the compound whose formula is Al2(SO4)3?
(Multiple Choice)
4.8/5  (33)
(33)
Which is a correct balanced chemical equation corresponding to the following description of a chemical reaction?
Hydrochloric acid reacts with magnesium metal to produce aqueous magnesium chloride and hydrogen gas.
(Multiple Choice)
4.8/5  (44)
(44)
The complete combustion of phenylhydrazine,C6H5NHNH2,with the oxidizer dinitrogen tetraoxide is shown in the following equation:
__ C6H5NHNH2 + __ N2O4 → __ CO2 + __ H2O + __ N2
When this equation is balanced,the sum of all the coefficients (using smallest whole numbers)is
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following has 62 neutrons,46 protons,and 46 electrons?
(Multiple Choice)
4.9/5  (35)
(35)
When the equation
__ (CH3)2NNH2 + __ N2O4 → __ N2 + __ H2O + __ CO2
Is balanced,the sum of all the coefficients (simplest whole number)is
(Multiple Choice)
4.9/5  (36)
(36)
The mass spectrum of an element with two naturally occurring isotopes is shown below.Its average atomic mass would be best estimated as 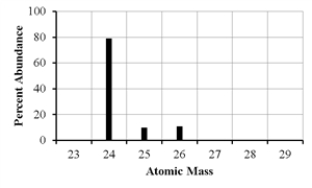
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following statements is true concerning the two nuclides 16O and 17O ?
(Multiple Choice)
5.0/5  (42)
(42)
Which of the following conclusions regarding Rutherford's gold foil experiment is not consistent with the observations?
(Multiple Choice)
4.8/5  (37)
(37)
Which formula is best described as a (condensed)structural formula?
(Multiple Choice)
4.9/5  (36)
(36)
Which combination of protons,neutrons,and electrons correctly represents a 57Fe nuclide?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 121 - 140 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)