Exam 2: Atoms, molecules, and Ions
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Treatment of sodium borohydride with sulfuric acid is a convenient method for the preparation of diborane:
__ NaBH4 + __ H2SO4 → __ B2H6 + __ H2 + __Na2SO4
When the equation is balanced,the lowest whole number coefficient for hydrogen is
(Multiple Choice)
4.8/5  (31)
(31)
Naturally occurring element X exists in three isotopic forms: X-28 (27.979 amu,92.21% abundance),X-29 (28.976 amu,4.70% abundance),and X-30 (29.974 amu,3.09% abundance).Calculate the atomic weight of X.
(Multiple Choice)
4.9/5  (41)
(41)
The names of the elements whose symbols are Si,P,Mn,and S are,respectively,
(Multiple Choice)
4.8/5  (40)
(40)
In the following chemical equation,what is the reactant?
CuSO4·5H2O(s)→ CuO(s)+ SO3(g)+ 5H2O(l)
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following atomic symbols represents an isotope of 94Mo?
(Multiple Choice)
4.9/5  (32)
(32)
In a particular mass of KAu(CN)2,there are 6.66 × 1020 atoms of gold.What is the total number of atoms in this sample?
(Multiple Choice)
4.8/5  (31)
(31)
All the following may change during a chemical reaction except
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements best describes the particulate representation depicted by the picture? 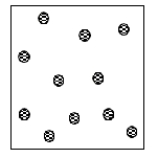
(Multiple Choice)
4.9/5  (35)
(35)
Suppose atom 1 has the same number of protons as atom 2,and atom 2 has the same number of neutrons as atom 3.Atom 1 does not have the same number of neutrons as atom 3.Which of the following statements is true?
(Multiple Choice)
4.9/5  (33)
(33)
What is the symbol of the nuclide having 13 protons and 14 neutrons?
(Multiple Choice)
5.0/5  (34)
(34)
What is the balanced chemical equation that represents the following reaction? 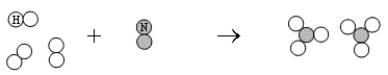
(Multiple Choice)
4.8/5  (28)
(28)
What is the subscript of potassium in the formula for potassium carbonate?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)