Exam 7: Quantum Theory of the Atom
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
When a particular metal is illuminated with photons,one electron is observed for each absorbed photon.What effect would decreasing the wavelength and number of photons have on the electrons leaving the surface?
(Multiple Choice)
4.8/5  (32)
(32)
The number of orbitals having a given value of l is equal to
(Multiple Choice)
4.9/5  (37)
(37)
A possible value of the magnetic quantum number ml for a 5p electron is
(Multiple Choice)
4.9/5  (34)
(34)
The electron in a hydrogen atom,originally in level n = 8,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron?
(c=3.00×108 m/s,h=6.63×10-34 J·s,RH=2.179×10-18 J)
(Multiple Choice)
4.9/5  (45)
(45)
What is the energy of a photon of electromagnetic radiation with a frequency of 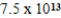 Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
Hz? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s)
(Multiple Choice)
4.9/5  (44)
(44)
The branch of physics that mathematically describes the wave properties of submicroscopic particles is called _____.
(Multiple Choice)
4.9/5  (36)
(36)
Consider the following energy-level diagram for a particular electron in an atom. 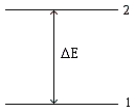 Based on this diagram,which of the following statements is incorrect?
Based on this diagram,which of the following statements is incorrect?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements is incorrect concerning the wave function?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 61 - 68 of 68
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)