Exam 7: Quantum Theory of the Atom
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Which of the following combinations of quantum numbers is permissible?
(Multiple Choice)
4.7/5  (37)
(37)
A laser emits photons having an energy of 3.74 × 10-19 J.What color would be expected for the light emitted by this laser? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J ⋅ s)
(Multiple Choice)
4.8/5  (33)
(33)
What is the frequency of photons that have molar energy of 339.00 kJ/mol?
(c = 3.00 × 108 m/s,h = 6.63 × 10-34 J·s,NA = 6.02 × 1023 mol-1)
(Multiple Choice)
4.9/5  (27)
(27)
What is the value of the angular momentum quantum number for an electron in a 1s orbital?
(Multiple Choice)
4.9/5  (40)
(40)
If the location of a particular electron can be measured only to a precision of 0.069 nm,what is the minimum uncertainty in the electron's velocity?
(me=9.109×10-31 kg,c=3.00×108 m/s,h=6.63×10-34 J·s)
(Multiple Choice)
4.8/5  (42)
(42)
Which quantum number distinguishes the different shapes of the orbitals?
(Multiple Choice)
4.8/5  (35)
(35)
The wavelength of a gamma ray is 4 × 10-11 m.Calculate the frequency of the ray.
(Multiple Choice)
4.9/5  (46)
(46)
Which hydrogen atom orbital has an energy essentially identical to a 3d orbital?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following scientists first postulated that the sharp lines in the emission spectra of elements were caused by electrons going from high-energy levels to low-energy levels?
(Multiple Choice)
4.9/5  (31)
(31)
What is the wavelength of photons that have molar energy of 515 kJ/mol? (c = 3.00 × 108 m/s,h = 6.63 × 10-34 J · s,NA = 6.02 × 1023 mol-1)
(Multiple Choice)
4.7/5  (42)
(42)
A radial probability plot for an electron in an atom,like that shown below, 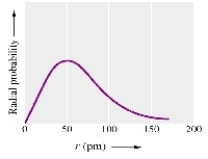
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following statements is a valid conclusion from the Heisenberg uncertainty principle?
(Multiple Choice)
4.8/5  (32)
(32)
Which type of electromagnetic radiation has the lowest energy?
(Multiple Choice)
5.0/5  (36)
(36)
What is the energy per mole of photons with a wavelength of 307.1 nm?
(c=3.00×108 m/s,h=6.63×10-34 J·s,NA=6.02×1023mol-1)
(Multiple Choice)
4.9/5  (40)
(40)
The square of the wave function,ψ2,of an electron in an atom
(Multiple Choice)
4.9/5  (47)
(47)
How many values are there for the magnetic quantum number when the value of the angular momentum quantum number is 3?
(Multiple Choice)
4.8/5  (36)
(36)
Which type of electromagnetic radiation has the lowest frequency?
(Multiple Choice)
4.9/5  (31)
(31)
What is the total number of subshells found in the n = 6 shell?
(Multiple Choice)
4.8/5  (43)
(43)
When an electron in an atom makes a transition from n = 4 to n = 5,which of the following statements is/are correct?
I.Energy is emitted.
II.Energy is absorbed.
III.The electron loses energy.
IV.The electron gains energy.
V.The electron cannot make this transition.
(Multiple Choice)
4.9/5  (36)
(36)
Showing 41 - 60 of 68
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)