Exam 9: Ionic and Covalent Bonding
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
For each of the following species except ____,the electronic structure may be adequately described by two resonance formulas.
(Multiple Choice)
4.8/5  (29)
(29)
Based on the following data,what is the I-I bond energy? 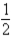 H2(g)+
H2(g)+ 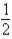 I2(g)→ HI(g); ΔH = 26.36 kJ
Bond
Bond Energy (kJ/mol)
H-H
435
H-I
295
I2(g)→ HI(g); ΔH = 26.36 kJ
Bond
Bond Energy (kJ/mol)
H-H
435
H-I
295
(Multiple Choice)
4.8/5  (39)
(39)
The total number of valence electrons in the phosphate ion is
(Multiple Choice)
4.8/5  (38)
(38)
Rank the following ions in order of decreasing atomic radii: Te2-,Te4+,Te6+.
(Multiple Choice)
4.9/5  (43)
(43)
Using bond-energy data,what is ΔH° for the following reaction?
CH3OH(g)+ H2S(g)→ CH3SH(g)+ H2O(g)
Bond
Bond Energy (kJ/mol)
C-H
413
C-O
358
O-H
463
C-S
259
S-H
339
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following is a correct Lewis electron-dot formula for CO?
(Multiple Choice)
4.9/5  (34)
(34)
How many valence electrons are there in the trifluoroacetate ion,CF3COO-?
(Multiple Choice)
4.8/5  (36)
(36)
The number of valence electrons in the propionate ion,CH3CH2COO-,is
(Multiple Choice)
4.7/5  (36)
(36)
Which one of the following has an enthalpy change that is equal to the lattice energy of SrCl2?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following Lewis structures best describes BF3?
(Multiple Choice)
4.8/5  (41)
(41)
In which of the following species is the octet rule violated by the central atom when the central atom has a formal charge of zero?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following is a correct description of lattice energy?
(Multiple Choice)
4.8/5  (41)
(41)
All of the following have ground-state noble-gas electron configurations except
(Multiple Choice)
4.7/5  (34)
(34)
Showing 21 - 40 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)