Exam 9: Ionic and Covalent Bonding
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
As the number of bonds between two carbon atoms increases,which of the following decrease(s)?
(Multiple Choice)
5.0/5  (42)
(42)
A bond in which an electron pair is unequally shared by two atoms is
(Multiple Choice)
5.0/5  (32)
(32)
Which pair of elements would form a covalent bond that is the least polar?
(Multiple Choice)
4.8/5  (37)
(37)
In the Lewis formula for phosphine,PH3,the number of lone pairs of electrons around the phosphorus atom is
(Multiple Choice)
4.9/5  (42)
(42)
The Lewis formula of which species does not represent an exception to the octet rule?
(Multiple Choice)
4.8/5  (30)
(30)
What is the total number of valence electrons in the ammonium ion,NH4+ ?
(Multiple Choice)
4.9/5  (31)
(31)
For the resonance hybrid of the nitrite ion, 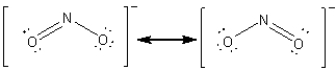 what is the average number of bonds between the nitrogen atom and an oxygen atom?
what is the average number of bonds between the nitrogen atom and an oxygen atom?
(Multiple Choice)
4.8/5  (37)
(37)
The Lewis structure for each of the following except ____contains at least one double bond.
(Multiple Choice)
4.9/5  (41)
(41)
Atoms of an element X have the ground-state electron configuration 1s22s22p63s23p4.What type of ion is X most likely to form?
(Multiple Choice)
4.8/5  (40)
(40)
During the formation of a chemical bond between two hydrogen atoms,which of the following statements is always true?
(Multiple Choice)
4.8/5  (35)
(35)
What is the ground-state electron configuration of the zinc ion,Zn2+?
(Multiple Choice)
4.9/5  (26)
(26)
In which of the following species is resonance most likely to take place?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following is the Lewis dot structure for the fluoride ion?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following diatomic molecules has the greatest bond energy?
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following bonds would be the least polar yet still be considered polar covalent?
(Multiple Choice)
4.8/5  (42)
(42)
Consider the reaction
2HCl(g)→ H2(g)+ Cl2(g); ΔH = 185 kJ
Which of the following statements is false?
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following compounds would be expected to have the lowest melting point?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 100 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)