Exam 13: Rates of Reaction
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
In the Arrhenius equation,the symbol A is called _____ and the symbol R is called _____.
(Multiple Choice)
5.0/5  (30)
(30)
One mechanism for the depletion of ozone in the stratosphere is proposed as follows:
Cl + O3 → ClO + O2
ClO + O → Cl + O2
Identify any catalysts and intermediates in the reaction.
(Multiple Choice)
4.8/5  (32)
(32)
A rate constant for a particular reaction is 0.0070 M-1·s−1.What is the overall order of this reaction?
(Multiple Choice)
4.7/5  (31)
(31)
A first-order chemical reaction is observed to have a rate constant of 24 min-1.What is the corresponding half-life for the reaction?
(Multiple Choice)
4.8/5  (32)
(32)
The rate constant for a reaction at 40.0°C is exactly 5 times that at 20.0°C.Calculate the Arrhenius energy of activation for the reaction.
(Multiple Choice)
4.9/5  (36)
(36)
The gas-phase decomposition of N2O5 is a first-order process with a rate constant of 1.50 × 10-3 s-1 at 55°C.The decomposition reaction is
N2O5(g)→ 2NO2(g)+ 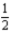 O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C)and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C)and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is not a postulate of collision theory?
(Multiple Choice)
4.8/5  (34)
(34)
The main reason for the increase in reaction rate with temperature is that
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following statements concerning the experimental determination of reaction rates is incorrect?
(Multiple Choice)
4.8/5  (38)
(38)
The complete mechanism for a reaction is considered to occur in two steps,one of which is slow and the other fast:
A + 2B → C + D slow
A + C → E + F fast
What is the rate law predicted by this mechanism?
(Multiple Choice)
4.7/5  (32)
(32)
Below is a proposed mechanism for the decomposition of H2O2.
H2O2 + I- → H2O + IO-
Slow
H2O2 + IO- → H2O + O2 + I-
Fast
Which of the following statements is incorrect?
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following statements is not a requirement for a valid reaction mechanism?
(Multiple Choice)
4.9/5  (38)
(38)
Two substances A and B react with each other in such a way that one-half of A remains after 25 min and one-fourth of A remains after 50 min.Doubling the concentration of B while keeping the concentration of A fixed doubles the rate of the reaction.This reaction is
(Multiple Choice)
4.8/5  (28)
(28)
A second-order reaction starts with an initial concentration of 0.100 mol/L of the reactant.If the rate constant is 2.4× 10-2 L/(mol ∙ s),what is the time required to decrease the initial concentration to 0.050 mol/L?
(Multiple Choice)
4.8/5  (37)
(37)
A mechanism that explains the rate law,Rate = k[(CH3)3CO2C(CH3)3],for the gas-phase thermal decomposition of di-tert-butyl peroxide is given below. ![A mechanism that explains the rate law,Rate = k[(CH<sub>3</sub>)<sub>3</sub>CO<sub>2</sub>C(CH<sub>3</sub>)<sub>3</sub>],for the gas-phase thermal decomposition of di-tert-butyl peroxide is given below. For this proposed mechanism,the rate-determining step(s)must be](https://storage.examlex.com/TB2288/11ea7a3a_9f0f_40b3_a82d_45143283fd5e_TB2288_00.jpg) For this proposed mechanism,the rate-determining step(s)must be
For this proposed mechanism,the rate-determining step(s)must be
(Multiple Choice)
4.7/5  (35)
(35)
The rate constants for the first-order decomposition of a compound are 5.22× 10-4 s-1 at 43°C and 2.91 × 10-3 s-1 at 62°C.What is the value of the activation energy for this reaction? (R = 8.31 J/(mol · K))
(Multiple Choice)
4.9/5  (29)
(29)
Given the following fast equilibrium (below)proposed for the gas phase reaction between NO and O2,what is the expression for the equilibrium concentration of the intermediate NO3?
NO + O2 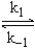 NO3
NO3
(Multiple Choice)
4.9/5  (37)
(37)
If the rate law for a reaction is
Rate = k[ClO3-][I-][H+]2
What are the units of k when the unit of time is seconds and the unit of concentration is moles per liter?
(Multiple Choice)
4.9/5  (33)
(33)
The balanced chemical equation and rate law for the reaction between NO(g)and H2(g)at a particular temperature are
2NO(g)+ 2H2(g)→ N2(g)+ 2H2O(g)
Rate = k[NO]2[H2]
What is the reaction order with respect to nitric oxide?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 41 - 60 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)