Exam 13: Rates of Reaction
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
The rate law for the chemical reaction
5Br-(aq)+ BrO3-(aq)+ 6H+(aq)→ 3Br2(aq)+ 3H2O(l)
Has been determined experimentally to be Rate = k[Br-][BrO3-][H+]2.What is the overall order of the reaction?
(Multiple Choice)
4.8/5  (33)
(33)
The reaction 3NO → N2O + NO2 is found to obey the rate law Rate = k[NO]2.If the first half-life of the reaction is found to be 2.0 s,what is the length of the fourth half-life?
(Multiple Choice)
4.8/5  (42)
(42)
The acid-catalyzed reaction of acetone,CH3COCH3,with iodine can be represented by the following net reaction:
CH3COCH3 + I2 ![The acid-catalyzed reaction of acetone,CH<sub>3</sub>COCH<sub>3</sub>,with iodine can be represented by the following net reaction: CH<sub>3</sub>COCH<sub>3</sub> + I<sub>2</sub> CH<sub>2</sub>ICOCH<sub>3</sub> + H<sup>+</sup> + I<sup>-</sup> It is found experimentally that the rate law for this reaction is Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][H<sup>+</sup>].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of](https://storage.examlex.com/TB2288/11ea7a3a_9f05_a39d_a82d_ff58c247adb6_TB2288_11.jpg) CH2ICOCH3 + H+ + I-
It is found experimentally that the rate law for this reaction is Rate = k[CH3COCH3][H+].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of
CH2ICOCH3 + H+ + I-
It is found experimentally that the rate law for this reaction is Rate = k[CH3COCH3][H+].Suppose that in trial 1,the initial rate of the reaction is measured with the initial concentrations of acetone,iodine,and hydrogen ion all equal to 0.10 M.Then,in trial 2,the initial rate of the reaction is measured with the initial concentrations all equal to 0.20 M.The initial rate of trial 2 will be larger than the initial rate of trial 1 by a factor of
(Multiple Choice)
4.9/5  (34)
(34)
When the concentrations of the reactants are increased,the rate of the reaction increases.This is best explained by
(Multiple Choice)
4.9/5  (34)
(34)
For the reaction
IO3-(aq)+ 5I-(aq)+ 6H+(aq)→ 3I2(aq)+ 3H2O(l)
The rate of disappearance of I O3-(aq)at a particular time and concentration is 2.5× 10-3 mol/(L · s).What is the rate of appearance of I2(aq)?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following reactions is not an example of homogeneous catalysis?
(Multiple Choice)
4.8/5  (43)
(43)
In a first-order reaction,the half-life is 139 minutes.What is the rate constant?
(Multiple Choice)
4.8/5  (42)
(42)
The rate constant for a first-order reaction is 1.5× 10-2 s-1 at 710 K and 4.1 × 10-2 s-1 at 884 K.What is the activation energy? (R = 8.31 J/(mol · K))
(Multiple Choice)
4.9/5  (35)
(35)
For the reaction
(CH3)3CCl(aq)+ OH-(aq)→ (CH3)3COH(aq)+ Cl-(aq)
It is found experimentally that doubling the initial concentration of (CH3)3CCl causes the initial reaction rate to double,but doubling the initial concentration of OH- has no effect on the rate.What is the rate law?
(Multiple Choice)
4.9/5  (40)
(40)
For the reaction
2N2O5(g)→ 4NO2(g)+ O2(g)
Which of the following expressions is equal to the rate of the reaction?
(Multiple Choice)
4.9/5  (42)
(42)
For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following experimental methods cannot be used to measure the rate of a reaction?
(Multiple Choice)
4.8/5  (37)
(37)
The OH· radical disproportionates according to the elementary chemical reaction 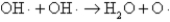 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following statements best describes the condition(s)needed for a successful formation for a product according to the collision model?
(Multiple Choice)
4.7/5  (33)
(33)
The reaction between selenous acid and the iodide ion in acid solution is
H2SeO3(aq)+ 6I-(aq)+ 4H+(aq)→ Se(s)+ 2I3-(aq)+ 3H2O(l)
The data in the following table were measured at 0°C.
Experiment
[H2SeO3]0 (M)
[H+]0 (M)
[I-]0 (M)
Initial Rate [mol/(L ∙ s)]
1
1)00 × 10-4
2)00 × 10-2
3)00 × 10-2
5)30 × 10-7
2
2)00 × 10-4
2)00 × 10-2
3)00 × 10-2
1)06 × 10-6
3
3)00 × 10-4
4)00 × 10-2
3)00 × 10-2
6)36 × 10-6
4
3)00 × 10-4
8)00 × 10-2
3)00 × 10-2
2)54 × 10-5
5
3)00 × 10-4
8)00 × 10-2
6)00 × 10-2
2)04 × 10-4
6
2)00 × 10-4
2)00 × 10-2
6)00 × 10-2
8)48 × 10-6
What is the rate constant for this reaction?
(Multiple Choice)
4.8/5  (29)
(29)
For which of the following hypothetical rate laws would the units of the rate constant have the general form M-3·time−1?
(Multiple Choice)
4.8/5  (35)
(35)
Ozone reacts with nitrogen dioxide to produce oxygen and dinitrogen pentoxide according to the following chemical equation:
O3(g)+ 2NO2(g)→ O2(g)+ N2O5(g)
The rate law for this reaction is Rate = k[O3][NO2].If concentration is measured in moles per liter and time is measured in seconds,what are the units of k?
(Multiple Choice)
4.8/5  (37)
(37)
If a reaction is first-order with respect to a particular reactant,when the concentration of that reactant is increased by a factor of 2,the reaction rate will _____.
(Multiple Choice)
4.8/5  (37)
(37)
For the hypothetical reaction aA → products,the concentration of A was monitored with time.Given the following graph of the experimental data,what is the rate constant for the loss of reactant A? 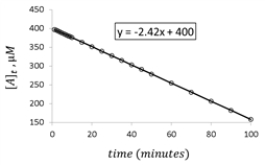
(Multiple Choice)
4.8/5  (34)
(34)
The rates of most chemical reactions are sensitive to a change in the temperature of the reaction system.The increase in rate as the temperature increases is best explained by
(Multiple Choice)
4.9/5  (28)
(28)
Showing 61 - 80 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)