Exam 13: Rates of Reaction
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
The nuclide 96Nb decays by a first-order process with a rate constant of 2.96 × 10-2 h-1.How long will it take for 86.0% of the initial amount of 96Nb to be consumed?
(Multiple Choice)
4.8/5  (47)
(47)
A possible mechanism for the gas phase reaction of NO and H2 is as follows:
Step 1
2NO 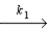 N2O2
Step 2
N2O2 + H2
N2O2
Step 2
N2O2 + H2 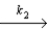 N2O + H2O
Step 3
N2O + H2
N2O + H2O
Step 3
N2O + H2 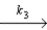 N2 + H2O
Which of the following statements concerning this mechanism is not directly supported by the information provided?
N2 + H2O
Which of the following statements concerning this mechanism is not directly supported by the information provided?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following is not a correct representation of the integrated rate expression for a first-order reaction?
(Multiple Choice)
4.9/5  (34)
(34)
At a given temperature,a first-order reaction has a rate constant of 2.5 × 10-3 s-1.How long will it take for the reaction to be 35% complete?
(Multiple Choice)
5.0/5  (29)
(29)
For the first-order reaction
1/2 N2O4(g)→ NO2(g); ΔH = 28.6 kJ
The rate constant is k = 3.24 × 105 s-1 at -7°C,and the activation energy is 53.7 kJ/mol.What is the rate constant at 26°C?
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following reactions is not an example of heterogeneous catalysis?
(Multiple Choice)
4.8/5  (41)
(41)
For the hypothetical reaction A → products,the concentration of A was monitored with time.From the following graph,what is the rate constant for the decomposition of A? 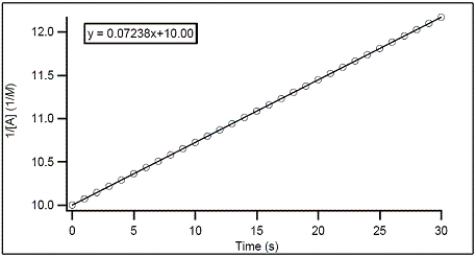
(Multiple Choice)
4.9/5  (40)
(40)
For the hypothetical reaction A → products,the concentration of A was monitored over time.From the following graph,what is the rate constant for the decomposition of A? 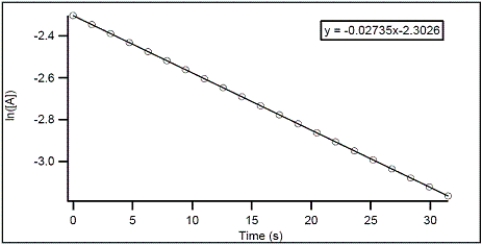
(Multiple Choice)
5.0/5  (45)
(45)
Which of the following corresponds to the correct integrated expression for a second-order reaction?
(Multiple Choice)
4.9/5  (42)
(42)
In the reaction 2H2O2(aq)→ 2H2O(l)+ O2(g),the initial concentration of H2O2 is 0.565 M and,17.0 seconds later,the concentration of H2O2 is 0.361 M.What is the average rate of reaction over this time interval?
(Multiple Choice)
4.9/5  (30)
(30)
The decomposition of ozone may occur through the two-step mechanism shown below:
Step 1
O3 → O2 + O
Step 2
O3 + O → 2O2
The oxygen atom is considered to be a(n)
(Multiple Choice)
4.8/5  (36)
(36)
For a certain first-order reaction with the general form aA → products,the rate is 0.32 M·s−1 when the concentration of the reactant is 0.27 M.What is the rate constant for this reaction?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 101 - 113 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)