Exam 17: Solubility and Complex-Ion Equilibria
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
What is the molar solubility of MgF2 in a 0.40 M NaF solution? For MgF2,Ksp = 8.4 × 10-8.
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following salts has the highest molar solubility in water?
(Multiple Choice)
4.8/5  (42)
(42)
Ksp for PbF2 is 4.0 ×10-8.If a 0.032 M NaF solution is saturated with PbF2,what is [Pb2+] in solution?
(Multiple Choice)
4.9/5  (47)
(47)
The figure below represents the result of adding which of the following aqueous solutions to a filtered,saturated solution of AgCl? 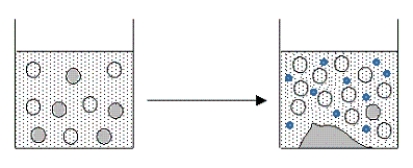
(Multiple Choice)
4.8/5  (41)
(41)
Cation C and anion A form an ionic compound for which Ksp = s2,where s is the molar solubility of the ionic compound.Which of Figures I-III represent(s)possible results of the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A? 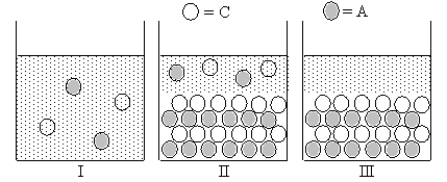
(Multiple Choice)
4.8/5  (42)
(42)
What is the molar equilibrium concentration of uncomplexed Fe2+(aq)in a solution composed of 1.4 mol Fe(CN)64− dissolved in 1.00 L of 0.33 M NaCN.Kf for Fe(CN)64− is 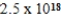 .
.
(Multiple Choice)
4.9/5  (44)
(44)
The solubility of calcium carbonate in water at 25°C is 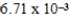 g/L.What is the Ksp of this sparingly soluble salt?
g/L.What is the Ksp of this sparingly soluble salt?
(Multiple Choice)
4.8/5  (38)
(38)
Pure water is saturated with slightly soluble calcium fluoride,CaF2.Which of the following is true concerning the equilibrium concentration of Ca2+?
(Multiple Choice)
4.8/5  (30)
(30)
The following reaction represents a step in the separation of which analytical group of cations?
Cu2+(aq)+ S2-(aq)→ CuS(s)
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following solutions should be added to a solution containing both copper(II)ions and silver(I)ions in order to precipitate only one of the ions?
(Multiple Choice)
4.9/5  (43)
(43)
In the qualitative analysis scheme for metal ions,how are the Analytical Group III cations separated from the cations of Analytical Groups IV and V?
(Multiple Choice)
4.8/5  (33)
(33)
Rank the following salts in order of increasing molar solubility.
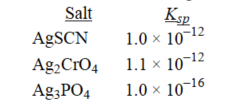
(Multiple Choice)
4.7/5  (28)
(28)
A(n)_____ is a Lewis base that bonds to a metal ion to form a complex ion.
(Multiple Choice)
4.8/5  (30)
(30)
What is the molar solubility of MgF2 in a 0.36 M Mg(NO3)2 solution? For MgF2,Ksp = 8.4 × 10-8.
(Multiple Choice)
4.7/5  (33)
(33)
The solubility of lead(II)sulfate is 4.0 × 10-2 g/L.What is the solubility product constant for lead(II)sulfate?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following salts has the lowest molar solubility in water?
(Multiple Choice)
4.7/5  (39)
(39)
Which sparingly soluble salt will exhibit the highest solubility at low pH's?
(Multiple Choice)
4.9/5  (43)
(43)
Suppose sodium hydroxide is added to a 0.0042 M solution of zinc nitrate such that the pH of the solution is 13.04.What is the equilibrium concentration of Zn2+?
Zn2+(aq)+ 4OH-(aq)  Zn(OH)42-(aq); Kf = 2.8 × 1015
Zn(OH)42-(aq); Kf = 2.8 × 1015
(Multiple Choice)
4.8/5  (37)
(37)
What is the solubility (in g/L)of barium chromate at 25°C? The solubility product constant for barium chromate is 1.2 × 10-10 at 25°C.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)