Exam 1: Matter: Its Properties and Measurement
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
A field is 100.0 yd long and 50.0 yd wide.If covered with water to a uniform depth of 2.00 in,what volume of water is present in U.S.gallons?
(Multiple Choice)
4.7/5  (30)
(30)
How many litres of wine can be held in a wine barrel whose capacity is 25.0 gal? 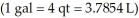
(Multiple Choice)
4.9/5  (46)
(46)
A 15.0 g sample of hydrated copper sulfate was heated to dryness and the new mass measured to be 9.59 g.Calculate the percentage of water in the hydrated crystal.Express your answer to the correct number of significant digits.
(Multiple Choice)
4.9/5  (43)
(43)
What is the volume (in cm3)of a 43.6 g piece of metal with a density of 2.71 g cm-3?
(Multiple Choice)
4.9/5  (40)
(40)
If the melting point of vanadium metal is 1910 °C,what is its melting point in kelvin?
(Multiple Choice)
4.8/5  (35)
(35)
If gasoline costs 98.5 cents per liter in Canada,what is the price in dollars per gallon?
(Multiple Choice)
4.7/5  (32)
(32)
Ethylene glycol has a density of 1.11 g/mL.What is the volume occupied by 30.0 g of ethylene glycol?
(Multiple Choice)
4.8/5  (30)
(30)
How many significant figures should the answer to the following calculation have?
(1.4312 - 1.1 × 10-2)÷ (1.0712 × 10-4)
(Multiple Choice)
4.8/5  (46)
(46)
A 2.0 gal flask weighs 4.0 lbs when empty.When it is filled with liquid,the flask weighs 4536.0 g.What is the density of the liquid in g/mL? (1 gal = 3.785 L,1 lb = 453.6 g)
(Multiple Choice)
4.7/5  (34)
(34)
A weatherman incorrectly stated that the temperature was 85 °F,35 °C.If the Fahrenheit temperature was correct,how far off was the Celsius temperature?
(Multiple Choice)
4.9/5  (31)
(31)
A fishing boat accidentally spills 3.0 barrels of diesel oil into the ocean.Each barrel contains 42 gallons.If the oil film on the ocean is 2.5 × 102 nm thick,how many square metres will the oil slick cover?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 121 - 136 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)