Exam 1: Matter: Its Properties and Measurement
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What mass of sugar,in grams,would be obtained from the evaporation of water from 1.5 kg of a sugar solution which is 10.5% sugar by mass?
(Multiple Choice)
4.9/5  (37)
(37)
The density of air under ordinary conditions at 25 °C is 1.19 g L-1.How many kilograms of air are in a room that measures 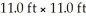 And has a 8.00 ft ceiling?
And has a 8.00 ft ceiling? 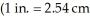 (exactly);
(exactly); 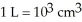 )
)
(Multiple Choice)
4.9/5  (26)
(26)
A physical property is the ability of a sample of matter to undergo a change in composition under certain conditions.
(True/False)
4.7/5  (44)
(44)
A block of wood has dimensions of 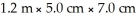 And has a mass of 3.0 kg.What is the density of the wood?
And has a mass of 3.0 kg.What is the density of the wood?
(Multiple Choice)
4.8/5  (41)
(41)
The diameter of an atom is approximately 1 × 10-10 m.What is the diameter in millimeters?
(Multiple Choice)
4.8/5  (38)
(38)
What is the mass of a 468 mL sample of ethanol? The density of ethanol is 0.789 g/mL.
(Multiple Choice)
4.8/5  (40)
(40)
What is the correct answer,with the proper number of significant figures,for the following calculation?
(1815 - 1806)× (9.11 × 7.92)
(Short Answer)
4.8/5  (35)
(35)
The mass of a proton is 1.67 × 10-27 kg.What is the mass of a proton in picograms?
(Multiple Choice)
4.8/5  (32)
(32)
The prefix "milli" and the prefix "micro" correspond,respectively,to the factors:
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the length of a steel cable (density 7.91 g/cm3)with a mass of 160.0 g and cross-sectional area of 0.500 cm2.
(Multiple Choice)
4.7/5  (32)
(32)
How many significant figures are there in the answer to the following problem? 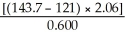
(Multiple Choice)
4.8/5  (42)
(42)
What is the answer to the correct number of significant figures of the following calculation? 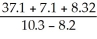
(Multiple Choice)
4.9/5  (37)
(37)
A theory is a model used to explain natural laws and make predictions about natural phenomena.
(True/False)
4.8/5  (40)
(40)
The estimated costs for remodelling the interior of an apartment are three 1-gallon cans of paint at $13.22 Each,two paint brushes at $9.53 Each,and $135 For a helper.The total estimated cost with the appropriate significant figures is $
(Multiple Choice)
4.8/5  (37)
(37)
Round the following number to four significant figures and express the result in standard exponential notation:
29,724.33
(Multiple Choice)
4.8/5  (41)
(41)
The result of addition can have more significant figures than any of the numbers added.
(True/False)
4.9/5  (29)
(29)
What is the answer,to the correct number of significant figures,of the following calculation? 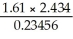
(Multiple Choice)
4.8/5  (33)
(33)
How many significant figures are there in the answer to the following problem?
(9.992 × 3.200)+ 0.610
(Multiple Choice)
4.8/5  (26)
(26)
Showing 41 - 60 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)