Exam 1: Matter: Its Properties and Measurement
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
A student weighed 30.00 μg of sulfur in the lab.This is the same mass as:
(Multiple Choice)
4.8/5  (30)
(30)
What is the answer to the correct number of significant figures of the following calculation? 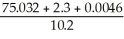
(Multiple Choice)
4.8/5  (36)
(36)
The average distance between nitrogen and oxygen atoms is 115 pm in a compound called nitric oxide.What is this distance in millimeters?
(Multiple Choice)
4.8/5  (30)
(30)
What mass,in g,of a solution containing 12% by mass sodium chloride is needed for a process that requires 8 g of sodium chloride?
(Multiple Choice)
4.9/5  (40)
(40)
A model that explains and makes predictions about natural phenomena is referred to as a:
(Multiple Choice)
5.0/5  (47)
(47)
Which of the following represents a base unit in the SI system of measurements?
(Multiple Choice)
4.7/5  (35)
(35)
A 16.0 g sample of iron has a volume of 2.035 cm3.What is its density expressed to the correct number of significant figures?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following numbers has the greatest number of significant figures?
(Multiple Choice)
4.8/5  (33)
(33)
Using the proper number of significant figures,how should the answer to the multiplication of the measurements 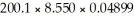 Be written?
Be written?
(Multiple Choice)
4.8/5  (31)
(31)
Without doing a calculation,find which of the following represents the highest temperature.
(Multiple Choice)
4.9/5  (38)
(38)
The mass of a single zinc atom is 1.086 × 10-22 g.This is the same mass as:
(Multiple Choice)
4.9/5  (33)
(33)
The scientific method is used to make theories that can no longer be changed.
(True/False)
4.8/5  (35)
(35)
Showing 21 - 40 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)