Exam 4: Chemical Reactions
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
If the density of ethanol,C2H5OH,is 0.789 g mL-1.How many millilitres of ethanol are needed to produce 15.0 g of CO2 according to the following chemical equation?
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
(Multiple Choice)
4.8/5  (40)
(40)
When the equation K2S2O3 + I2 → K2S4O6 + KI is balanced with the smallest integer coefficients,the coefficient of KI is:
(Multiple Choice)
4.8/5  (31)
(31)
Given the reaction:
P4(l)+ 6 Cl2(g)→ 4 PCl3(l)
If the percent yield is 82%,what mass of P4 is required to obtain 2.30 g PCl3 (Cl2 in excess)?
(Multiple Choice)
4.8/5  (36)
(36)
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
PCl3(l)+ Cl2(g)+ P4O10(s)→ POCl3(l)
(Multiple Choice)
4.8/5  (29)
(29)
If 0.500 mol of CaCl2 is mixed with 0.200 mol Na3PO4,the maximum amount in moles of Ca3(PO4)2 that can be formed is 0.10 moles.Calculate the extent of reaction ,x.
(Multiple Choice)
4.9/5  (37)
(37)
In the following reaction:
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
14.0 g KClO3 yielded 1.40 g KCl.What is the percent yield?
(Multiple Choice)
4.7/5  (38)
(38)
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
H2(g)+ O2(g)→ H2O(l)
(Multiple Choice)
4.8/5  (44)
(44)
In a balanced equation,the number of atoms of each element in the products must equal to the number of atoms of each element in the reactants.
(True/False)
4.8/5  (36)
(36)
Consider the gaseous reaction:
N2H4(g)+ 3 O2(g)→ 2 NO2(g)+ 2 H2O(g)
If the above reaction has a percent yield of 98.5%,what mass in grams of oxygen is needed to produce 49.0 g of NO2(g),assuming an excess of N2H4?
(Multiple Choice)
4.8/5  (41)
(41)
The chemical reaction occurring during the discharge of a lead storage battery can be represented by the equation:
Pb(s)+ PbO2(s)+ 2 H2SO4(aq)→ 2 PbSO4(s)+ 2 H2O(l)
Which is the limiting reagent and the amount of PbSO4 produced if 39.8 g of Pb,57.9 g of PbO2, and 352 mL of 0.375 M solution of H2SO4 is used?
(Multiple Choice)
4.9/5  (36)
(36)
If an aqueous solution containing 46 g of sodium carbonate per liter is mixed with an equal volume of 0.20 M aqueous hydrochloric acid,what would be the molarity of sodium chloride in the final solution,assuming volumes were additive?
(Multiple Choice)
4.9/5  (49)
(49)
If 5.97 mL of a solution of NaCl contains 2.54 mg of sodium ion,what is the molarity of the sodium chloride solution?
(Multiple Choice)
4.9/5  (42)
(42)
How many mL of 0.024 M solution can be produced from 14.1 mL of 3.0 M solution?
(Multiple Choice)
4.8/5  (40)
(40)
"Washing soda" (sodium carbonate)may be used to "soften" water by the removal of certain ions that would otherwise react with common soaps.When the "hardness" is due to calcium ion,the "softening" process may be represented as:
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
What mass of sodium carbonate would be required to remove essentially all of the calcium ion from 750 L of solution containing 43 mg Ca2+ per liter?
(Multiple Choice)
4.7/5  (37)
(37)
How many millilitres of a stock solution of 11.1 mol L-1 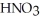 (aq) would be needed to prepare 0.500 L of 0.500 mol L-1
(aq) would be needed to prepare 0.500 L of 0.500 mol L-1 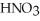 ?
?
(Multiple Choice)
4.9/5  (38)
(38)
Gases emitted during volcanic activity often contain high concentrations of hydrogen sulfide and sulfur dioxide.These gases may react to produce deposits of sulfur according to the equation: 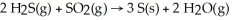 For the complete reaction of 6.41 mol of hydrogen sulfide:
For the complete reaction of 6.41 mol of hydrogen sulfide:
(Multiple Choice)
4.7/5  (42)
(42)
You have 10.00 L of a 0.350 M KCl solution,but you need a solution that is 0.450 M.What volume of water,in L,would you evaporate from the solution?
(Multiple Choice)
4.7/5  (45)
(45)
How many grams of CrSO4 will be made from 25.0 grams each of Zn,K2Cr2O7,and H2SO4?
4 Zn + K2Cr2O7 + 7 H2SO4 → 4 ZnSO4 + 2 CrSO4 + K2SO4 + 7 H2O
(Multiple Choice)
4.8/5  (32)
(32)
What mass of oxygen gas would be consumed by the complete combustion of 7.5 g of a mixture of propane (C3H8)and butane (C4H10)in the mole ratio of 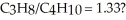
(Multiple Choice)
4.9/5  (33)
(33)
What is the stoichiometric coefficient for oxygen when the following equation is balanced using the lowest,whole-number coefficients?
________ CH14O(l)+ ________ O2(g)→ ________ CO2(g)+ ________ H2O(l)
(Multiple Choice)
4.9/5  (45)
(45)
Showing 141 - 160 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)