Exam 4: Chemical Reactions
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
What mass of water is produced in the metathesis reaction 75.2 g Zn(OH)2 with 43.4 g HCl?
(Multiple Choice)
4.9/5  (45)
(45)
What is the concentration (M)of a NaCl solution prepared by dissolving 7.2 g of NaCl in sufficient water to give 425 mL of solution?
(Short Answer)
5.0/5  (40)
(40)
Consider the equation:
2 Na + 2 H2O → 2 NaOH + H2
If 92.0 g of sodium is reacted with 76.0 g of water until the reaction goes to completion,which reactant will remain and in what quantity?
(Multiple Choice)
4.8/5  (34)
(34)
How many grams of sulfuric acid,H2SO4,can be obtained from 400 grams of iron ore if the ore is 80.0% by mass FeS? The reactions involved are given below.
4 FeS + 7O2 → 2 Fe2O3 + 4 SO2
2 SO2 + O2 → 2 SO3
SO3 + H2O → H2SO4
(Multiple Choice)
4.8/5  (32)
(32)
Given the reaction:
2 KMnO4 + 10 KI + 8 H2SO4 → 6 K2SO4 + 2 MnSO4 + 5 I2 + 8 H2O
How many moles of I2 are produced by reacting 28.0 g KMnO4,18.0 g KI,and 46.0 g H2SO4?
(Multiple Choice)
4.8/5  (35)
(35)
The numbers in front of formulas in balanced equations are called stoichiometric coefficients.
(True/False)
4.7/5  (41)
(41)
Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.How many grams of oxygen are formed when 10.0 g of dinitrogen monoxide decomposes?
(Multiple Choice)
4.9/5  (35)
(35)
When the equation CS2 + Cl2 → CCl4 + S2Cl2 is balanced with the smallest integer coefficients,the sum of the coefficients is:
(Multiple Choice)
4.9/5  (44)
(44)
Our task is to measure the volume of blood in an elephant.One way to do this would be to drain its blood into a suitable container.Aside from harmful side effects which this method has on the elephant,it will not be successful,as blood will still remain behind in the tissues.Hence,we will inject 2.00 ml of a 2.00 M solution of a dye which the elephant will not appreciably metabolize or excrete in one hour and then measure the concentration of this dye in the bloodstream (our sample being taken from another leg than the point of injection)after 30 minutes,a sufficient time to thoroughly mix the dye in the bloodstream.The concentration of dye at this point is 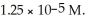 What is the volume of blood in the elephant?
What is the volume of blood in the elephant?
(Multiple Choice)
4.9/5  (44)
(44)
How many moles of CuO can be produced from 0.900 mol of Cu2O in the following reaction?
2 Cu2O(s)+ O2(g)→ 4 CuO(s)
(Multiple Choice)
4.7/5  (38)
(38)
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide:
2 Mg(s)+ O2(g)→ 2 MgO(s)
How many moles of O2 Are consumed when 0.770 mol of magnesium burns?
(Multiple Choice)
4.8/5  (35)
(35)
How many millilitres of 0.200 mol L-1 FeCl3(aq)are needed to react with an excess of Na2S(aq)to produce 1.38 g of Fe2S3 if the percent yield for the reaction is 65.0%?
3 Na2S(aq)+ 2 FeCl3(aq)→ Fe2S3(s)+ 6 NaCl(aq)
(Multiple Choice)
4.8/5  (47)
(47)
To measure the volume of an irregularly shaped container filled with water,1.00 mL of 2.00 M potassium chloride solution is added.After stirring,a 5.00 mL sample was removed from the container and found to contain 2.54 × 10-2 mg of potassium ion.What is the volume of the container?
(Multiple Choice)
4.9/5  (35)
(35)
What mass of MgCl2 in grams must be added to 250.0 mL of a 0.25 M MgCl2 solution to produce a 0.40 M solution,assuming no change of volume upon addition?
(Multiple Choice)
5.0/5  (43)
(43)
Pure acetic acid ( CH3COOH)is a liquid and is known as glacial acetic acid.Calculate the molarity of a solution prepared by dissolving 10.00 mL of glacial acetic acid at 25 °C in sufficient water to give 500.0 mL of solution.The density of glacial acetic acid at 25 °C is 1.05 g mL-1.
(Multiple Choice)
4.8/5  (35)
(35)
When the equation Fe2(C2O4)3 → FeC2O4 + CO2 is balanced with the smallest integer coefficients,the coefficient of CO2 is:
(Multiple Choice)
4.7/5  (34)
(34)
What is the concentration of an AlCl3(aq) solution if 150 mL of the solution contains 450 mg of Cl- ion?
(Multiple Choice)
4.8/5  (39)
(39)
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
C2H6 + O2 → CO2 + H2O
(Multiple Choice)
4.8/5  (32)
(32)
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
Al + Fe2O3 → Al2O3 + Fe
(Multiple Choice)
4.9/5  (34)
(34)
Showing 81 - 100 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)