Exam 4: Chemical Reactions
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
A formula is a shorthand way of representing a chemical reaction.
(True/False)
4.9/5  (40)
(40)
How many grams of AgNO3 are needed to make 250 mL of an aqueous solution that is 0.135 mol L-1?
(Multiple Choice)
4.9/5  (47)
(47)
What volume of concentrated acetic acid  Is needed to prepare 250 mL of a 0.30 M aqueous solution?
Is needed to prepare 250 mL of a 0.30 M aqueous solution?
(Multiple Choice)
4.8/5  (36)
(36)
Identify the coefficient of NO(g)when the following reaction is balanced,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25 °C.The density of nitrogen monoxide at 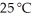 Is 1.23 g L-1.
(unbalanced)NH3(g)+ O2(g)→ NO(g)+ H2O(l)
Is 1.23 g L-1.
(unbalanced)NH3(g)+ O2(g)→ NO(g)+ H2O(l)
(Multiple Choice)
4.8/5  (43)
(43)
A student prepared a stock solution by dissolving 10.0 g of KOH in enough water to make 150 mL of solution.She then took 15.0 mL of the stock solution and diluted it with enough water to make 65.0 mL of a final solution.What is the concentration of KOH for the final solution?
(Multiple Choice)
4.9/5  (40)
(40)
The Haber Process for the production of ammonia is represented by:
3 H2(g)+ N2(g)→ 2 NH3(g)
If a mixture of 30 g of hydrogen with 10 g of nitrogen produced 8.4 g of ammonia,what was the percent yield?
(Multiple Choice)
4.9/5  (35)
(35)
Lithium and nitrogen react in a combination reaction to produce lithium nitride:
6 Li(s)+ N2(g)→ 2Li3N(s)
In a particular experiment,3.50 g samples of each reagent are reacted.What is the theoretical yield of lithium nitride?
(Multiple Choice)
4.7/5  (36)
(36)
Given the reaction:
2KMnO4 + 10 KI + 8 H2SO4 → 6 K2SO4 + 2 MnSO4 + 5 I2 + 8 H2O
How many moles of H2SO4 are required to produce 2.0 moles of I2,given the other reactants are in excess?
(Multiple Choice)
5.0/5  (43)
(43)
What is the molarity of methanol, 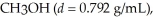 If 150.0 mL is dissolved in enough water to make 4.00 L of solution?
If 150.0 mL is dissolved in enough water to make 4.00 L of solution?
(Multiple Choice)
4.8/5  (34)
(34)
What is the sum of the coefficients when the following is balanced with the smallest integer coefficients?
Al(s)+ HCl(aq)→ AlCl3(aq)+ H2(g)
(Multiple Choice)
4.8/5  (44)
(44)
How many grams of CH3OH must be added to water to prepare 150 mL of a solution that is 2.0 mol L-1 CH3OH?
(Short Answer)
5.0/5  (33)
(33)
What are the smallest,whole-number coefficients in front of NO3-(aq)and Zn(s)when the following redox equation is balanced? The reaction occurs in acidic solution.
________ NO3-(aq)+ ________ Zn(s)→ ________ NO(g)+ ________ Zn2+(aq)?
(Multiple Choice)
4.9/5  (41)
(41)
A stock solution of 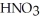 Is prepared and found to contain 13.5 mol L-1 of
Is prepared and found to contain 13.5 mol L-1 of 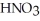 (aq).If 25.0 mL of the stock solution is diluted with water to a final volume of 0.500 L,what is the concentration of the diluted solution?
(aq).If 25.0 mL of the stock solution is diluted with water to a final volume of 0.500 L,what is the concentration of the diluted solution?
(Multiple Choice)
4.8/5  (36)
(36)
The molarity of a solution that contains 14.7 g of H2SO4 in 200.0 mL solution is ________.
(Multiple Choice)
4.8/5  (36)
(36)
What is the molarity of formaldehyde in a solution containing 0.25 g of formaldehyde (CH2O)per mL?
(Multiple Choice)
5.0/5  (40)
(40)
What is the percent yield if 122 grams of SiO2 are made from 246 g of Cr2O3 by the following equation?
3 Si(s)+ 2 Cr2O3(s)→ 3 SiO2(s)+ 4 Cr(l)
(Multiple Choice)
4.9/5  (34)
(34)
If 0.500 mol of CaCl2 is mixed with 0.200 mol Na3PO4,the maximum amount in moles of Ca3(PO4)2 that can be formed is:
(Multiple Choice)
4.8/5  (33)
(33)
Cryolite is a compound needed for the Hall-Heroult process for producing aluminum.Cryolite is produced by the following reaction:
6 HF + Al(OH)3 + 3 NaOH → Na3AlF6 + 6 H2O
How many grams of cryolite are produced if the reaction has a 67.3% yield and a limiting reagent of 35.4 grams of NaOH?
(Multiple Choice)
4.8/5  (33)
(33)
Acetylene gas may be produced by carefully adding water to calcium carbide,according to the equation:
CaC2(s)+ 2 H2O(l)→ Ca(OH)2(s)+ C2H2(g)
When a 37 g sample of an impure calcium carbide was treated with excess water,13 g of acetylene gas was produced.If the reaction is essentially 100% efficient,what was the percentage of nonreacting impurity in the carbide sample?
(Multiple Choice)
4.8/5  (41)
(41)
Calculate the concentration (M)of sodium ions in a solution made by diluting 40.0 mL of a 0.474 mol L-1 of an aqueous solution of sodium sulfide with water to a total volume of 300 mL.
(Short Answer)
4.8/5  (34)
(34)
Showing 61 - 80 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)